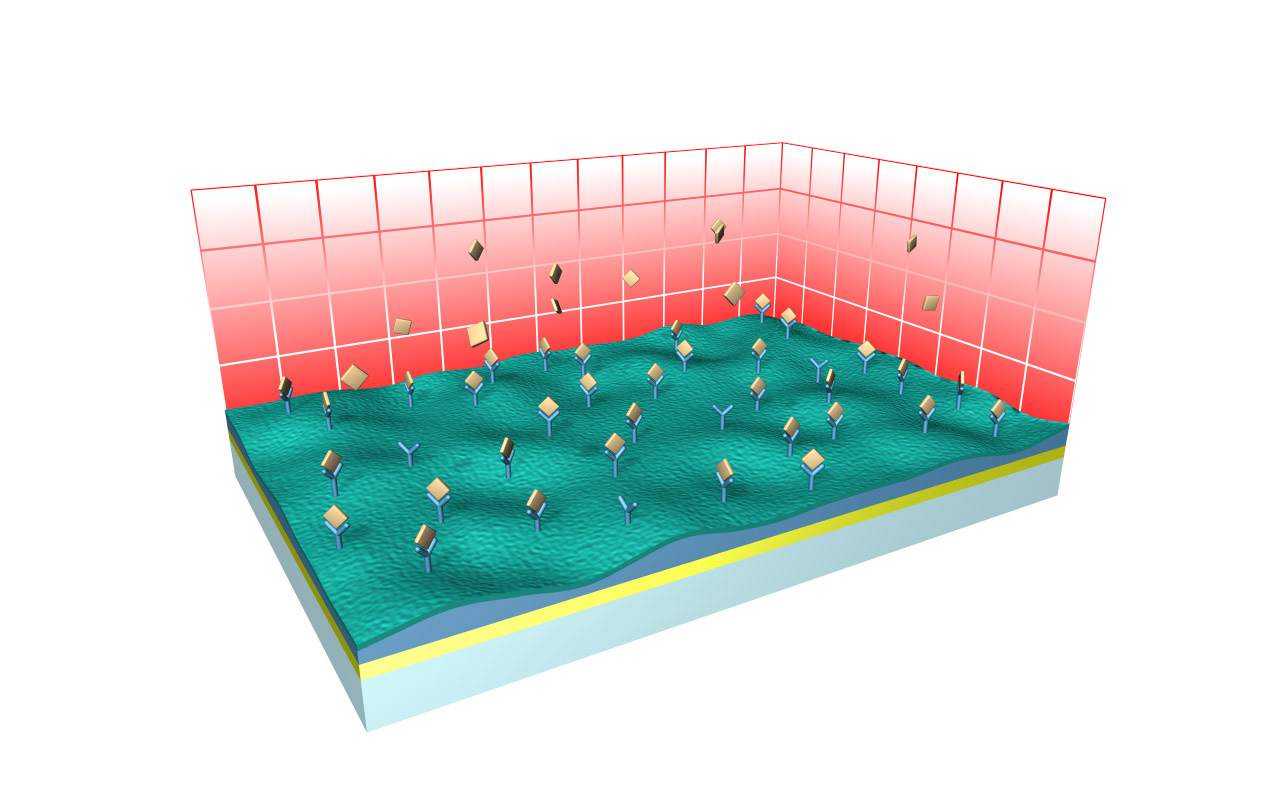
CMD–modified sensor chips
XanTec’s CMD sensor chips are based on a 2D (CMDP) or 3D hydrogel matrix consisting of carboxymethyl-dextran chains grafted onto a hydrophilic adhesion promoter on a gold support. Ligands can be covalently attached through their amine, thiol, or aldehyde groups using established coupling chemistries such as EDC/NHS activation, thiol-maleimide coupling, or reductive amination. This versatility enables the immobilization of a wide spectrum of biomolecules including proteins, antibodies, peptides, nucleic acids, and small organic compounds.
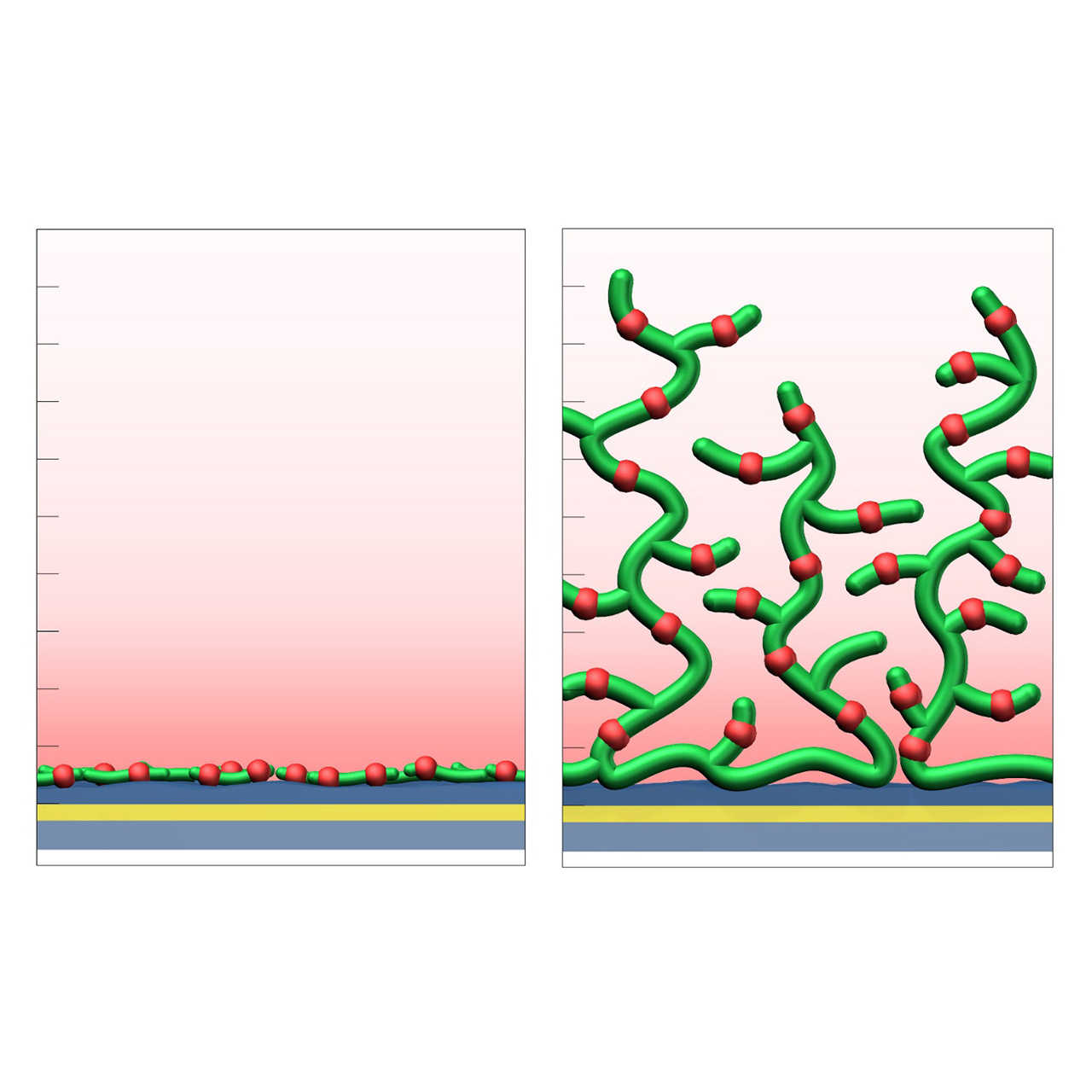
The CMD sensor chip portfolio spans electrostatic immobilization capacities from a few thousand μRIU (CMDP) to about 50,000 μRIU (CMD700M), covering analytes from large viruses to small organic fragments. Owing to this versatility, CMD chips are used in biochemical research, assay development, quality control, trace analysis, and drug discovery. Different chain densities further enhance flexibility:
- M variants (medium density, e.g., CMD200M, CMD700M) enable high ligand loading and elevated sensitivity for small-molecule kinetics and fragment screening.
- L variants (low density, e.g., CMD50L, CMD200L) occupy less of the evanescent field, minimize diffusion effects, and are ideal for capture assays or large analytes.
Key features:
- Versatile ligand coupling: Covalent coupling through amine, thiol, or aldehyde groups via standard chemistries (EDC/NHS, maleimide, reductive amination).
- Wide immobilization range: From several thousand to ≈ 50,000 μRIU, suitable for analytes from whole cells and viruses to fragments < 300 Da.
- Bioinert nanoarchitecture: Proprietary hydrophilic adhesion promoter combined with a hydrated CMD matrix minimizes nonspecific binding and matrix-analyte interactions.
- Application versatility: Suitable for kinetic, equilibrium, and concentration analyses, as well as diverse screening applications in drug discovery.
- High chemical stability: Withstands typical regeneration conditions, maintaining consistent response levels and kinetic behavior after multiple regeneration cycles.
| Product code 2 | CMDP | CMD50L | CMD200L | CMD200M | CMD700M |
|---|---|---|---|---|---|
| Base coating | 2D, ultra-short bioinert CM-dextran (high density) | 3D, 50 nm bioinert CM-dextran (low density) | 3D, 200 nm bioinert CM-dextran (low density) | 3D, 200 nm bioinert CM-dextran (medium density) | 3D, 700 nm bioinert CM-dextran (medium density) |
| Immobilization capacity [µRIU] 2 | ≈ 5,000 | ≈ 10,000 | ≈ 22,000 | ≈ 33,000 | ≈ 50,000 |
| Recommended ligands |
|
||||
| Recommended analytes |
|
|
|
|
|
| Intended purpose |
|
|
|
|
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 This overview represents a selection of the full CMD sensor chip portfolio.
3 Preconcentration capacity determined by injecting 100 µg/mL bovine serum albumin (BSA) in 5 mM sodium acetate pH 5.0, with 1 µRIU corresponding approximately to 1 RU. Maximum covalent coupling yields can vary and depend strongly on the properties of the protein to be immobilized. Under optimal conditions, typical coupling efficiencies range from approximately 20–45% of the respective electrostatic preconcentration capacity, with acidic proteins generally exhibiting lower coupling efficiencies.
HC–modified sensor chips
XanTec’s HC sensor chips are based on a 2D (HCP) or 3D hydrogel matrix composed of highly flexible, bioinert polycarboxylate chains grafted onto a hydrophilic adhesion promoter on a gold support. Ligands can be covalently attached through their amine, thiol, or aldehyde groups using established coupling chemistries such as EDC/NHS activation, thiol-maleimide coupling, or reductive amination. This versatility enables the immobilization of a wide range of biomolecules including proteins, antibodies, peptides, nucleic acids, carbohydrates, and small organic compounds.
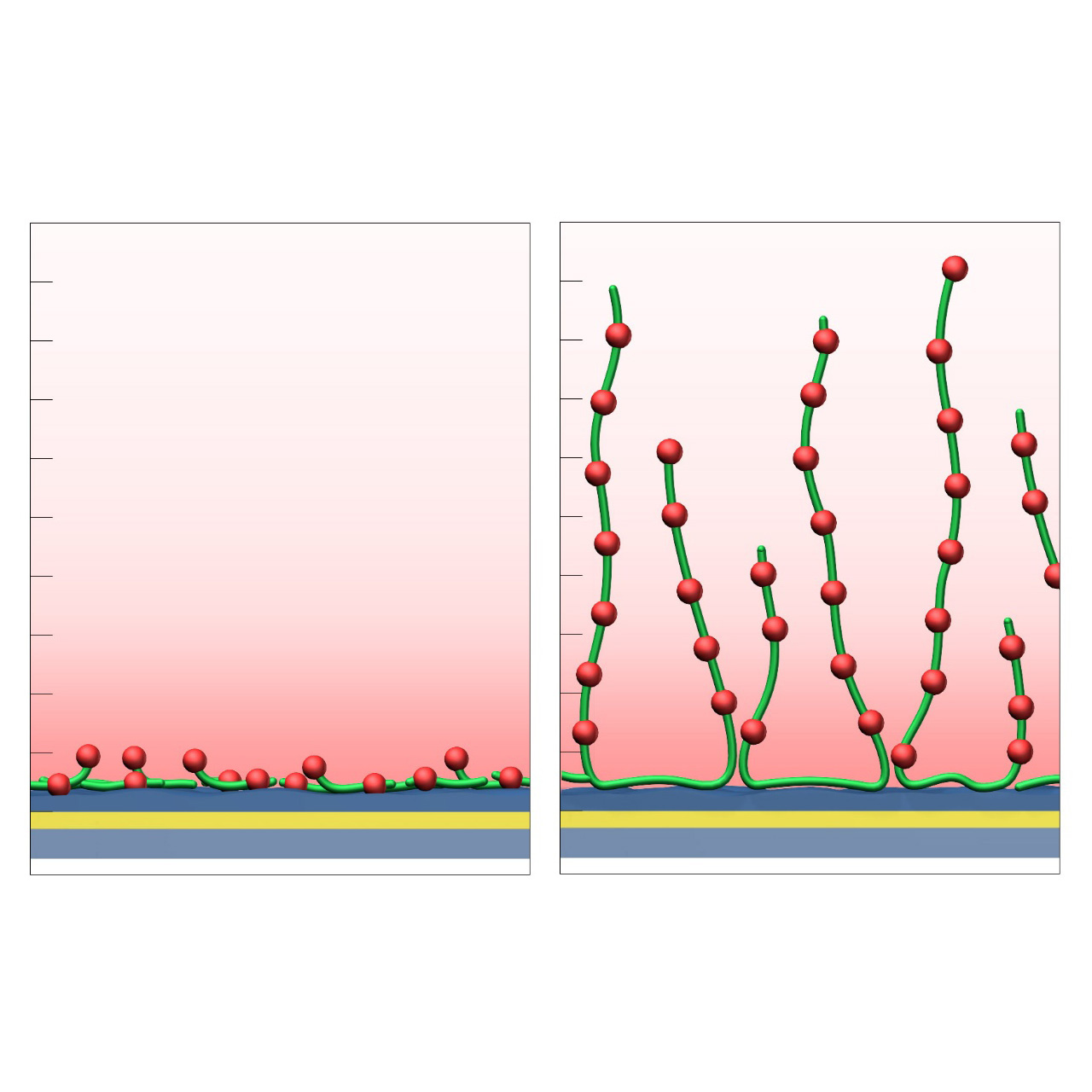
The HC sensor chip portfolio spans electrostatic immobilization capacities from a few thousand μRIU (HCP) to ≈ 55,000 μRIU (HC1500M), covering analytes from large viruses to small organic fragments. The HC polycarboxylate matrix is more flexible and less bulky than carboxymethyl-dextran coatings, occupying a smaller fraction of the evanescent field. Its strongly hydrated polymer brush makes the surface highly bioinert and minimizes nonspecific binding. This combination provides high immobilization capacity with excellent diffusion properties, making HC chips a preferred alternative to CMD-based sensor surfaces in biochemical research, assay development, quality control, trace analysis, and drug discovery.
Key features:
- Versatile ligand coupling: Covalent coupling through amine, thiol, or aldehyde groups via standard chemistries (EDC/NHS, maleimide, reductive amination).
- Wide immobilization range: From several thousand to ≈ 55,000 μRIU, suitable for analytes from whole cells and viruses to fragments < 300 Da.
- Bioinert nanoarchitecture: Proprietary hydrophilic adhesion promoter combined with a strongly hydrated polycarboxylate matrix minimizes nonspecific binding.
- Application versatility: Suitable for kinetic, equilibrium, and concentration analyses, as well as diverse screening applications in drug discovery.
- High chemical stability: Withstands typical regeneration conditions, maintaining consistent response levels and kinetic behavior after multiple regeneration cycles.
- No polysaccharide backbone: Lack of carbohydrate motifs prevents unwanted interactions with lectins or carbohydrate-binding proteins, making HC sensor chips ideal for analyzing this class of biomolecules and bioelectrical impedance analysis (BIA) of carbohydrates.
| Product code 2 | HCP | HC30M | HC200M | HC1500M |
|---|---|---|---|---|
| Base coating | 2D, ultra-short bioinert polycarboxylate (high density) | 3D, 30 nm bioinert polycarboxylate (medium density) | 3D, 200 nm bioinert polycarboxylate (medium density) | 3D, 1500 nm bioinert polycarboxylate (medium density) |
| Immobilization capacity [µRIU] 3 | ≈ 5,000 | ≈ 19,000 | ≈ 33,000 | ≈ 55,000 |
| Recommended ligands |
|
|||
| Recommended analytes |
|
|
|
|
| Intended purpose |
|
|
|
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 This overview represents a selection of the full HC sensor chip portfolio.
3 Preconcentration capacity determined by injecting 100 µg/mL bovine serum albumin (BSA) in 5 mM sodium acetate pH 5.0, with 1 µRIU corresponding approximately to 1 RU. Maximum covalent coupling yields can vary and depend strongly on the properties of the protein to be immobilized. Under optimal conditions, typical coupling efficiencies range from approximately 20–45% of the respective electrostatic preconcentration capacity, with acidic proteins generally exhibiting lower coupling efficiencies.
NeutrAvidin–modified sensor chips
XanTec NeutrAvidin-modified sensor chips are coated with a bioinert, charge-reduced (poly)carboxylate matrix (HLC), pre-functionalized with a recombinant 52 kDa NeutrAvidin tetramer. This immobilized NeutrAvidin efficiently captures biotinylated biomolecules—including proteins, nucleic acids, peptides, and other biotin-tagged ligands—under physiological conditions.
NeutrAvidin-modified sensor chips are especially tailored to reduce nonspecific binding in SPR assays. This is achieved by combining the deglycosylated, nearly charge-neutral (under physiological conditions) NeutrAvidin, with the highly bioinert, charge-reduced HLC coating, where most negative charges are neutralized. This unique combination renders the NAHLC sensor chips particularly suitable for assessing demanding analytes with high positive net charge.
Due to the exceptionally high binding affinity of NeutrAvidin for biotin (dissociation equilibrium constant ≈ 10⁻¹⁵ M), XanTec’s NAHLC sensor chips allow stable immobilization of biotinylated ligands with negligible dissociation.
Key features:
- Reduced nonspecific binding: NAHLC is an alternative to streptavidin-modified sensor chips in assays affected by nonspecific binding.
- Fast assay development: No preconcentration or surface activation required. Controlled ligand immobilization is performed under physiological conditions, reducing preparation time and ensuring reproducibility.
- Exceptional stability: Exceptionally strong NeutrAvidin-biotin binding makes this surface compatible with most common regeneration protocols.
- Oriented immobilization: Controlled biotinylation via biomolecular tools such as AviTag allows for maximum ligand activity and reproducible immobilization results.
- Broad application range: Ideal for immobilization of a wide variety of biotinylated ligands, making it suitable for protein interaction studies, nucleic acid hybridization, antibody screening, and other biosensing applications.
- Versatile capture capacity: Available on various HLC base coatings, offering different capture capacities to match specific experimental needs.
| Product code | NAHCP 2 | NAHLC30M | NAHLC200M | NAHLC1500M |
|---|---|---|---|---|
| Base coating | 2D, ultra-short bioinert polycarboxylate (medium density) | 3D, 30 nm bioinert polycarboxylate (medium density, reduced charge) | 3D, 200 nm bioinert polycarboxylate (medium density, reduced charge) | 3D, 1500 nm bioinert polycarboxylate (medium density, reduced charge) |
| Capture immobilization capacity [µRIU] 3 | ≈ 800 | ≈ 1,700 | ≈ 3,300 | ≈ 4,000 |
| Recommended ligands | biotinylated
|
|||
| Recommended analytes |
|
|
|
|
| Intended purpose |
|
|
|
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 NAHCP is the only variant that employs the classic HC matrix instead of HLC.
3 Based on specific binding of 100 µg/mL biotinylated bovine serum albumin (BSA) in phosphate-buffered saline (PBS), with 1 µRIU corresponding approximately to 1 RU.
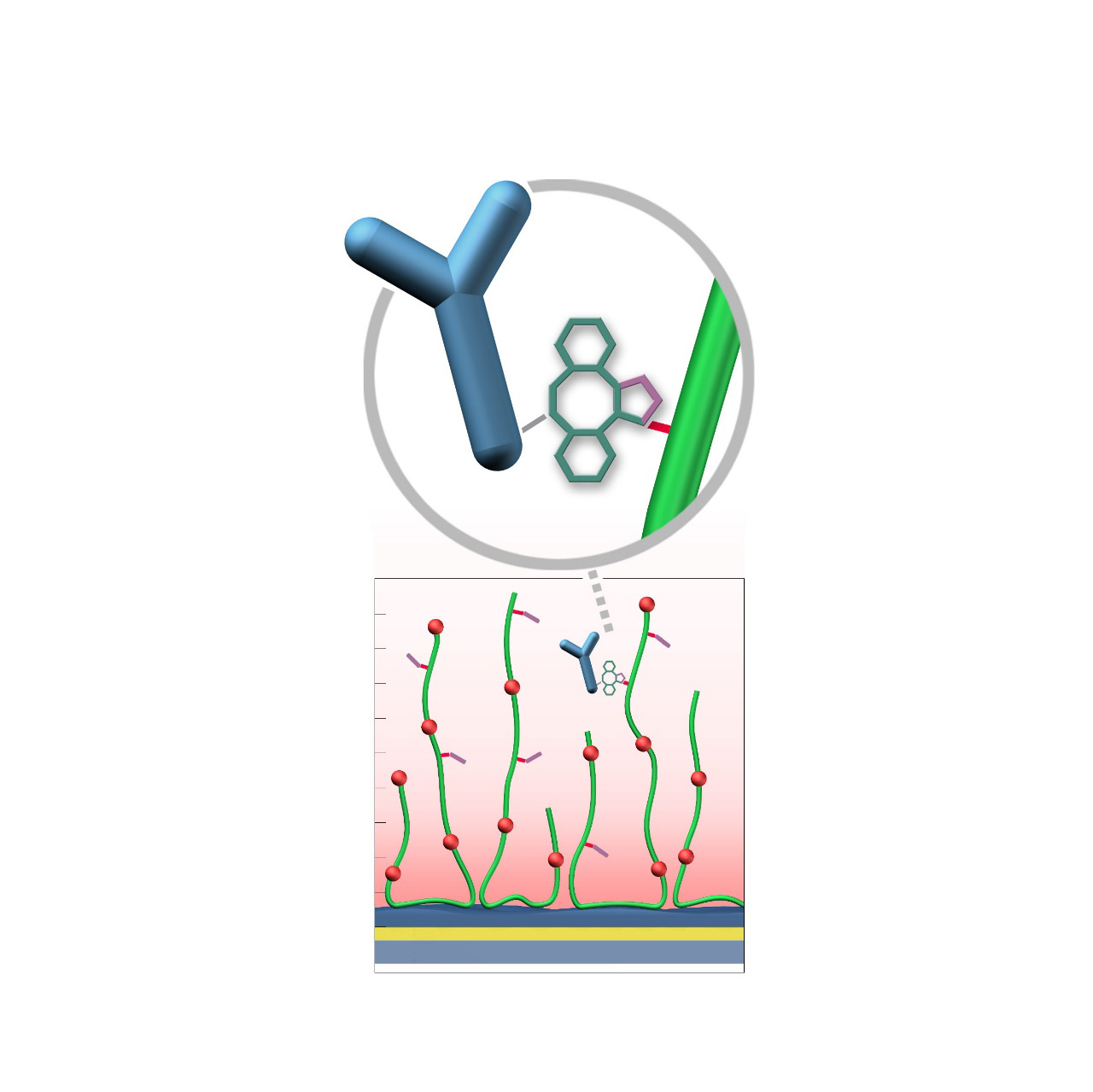
Azide–modified sensor chips
XanTec’s AZ sensor chips are coated with a bioinert polycarboxylate matrix (CMD or HC), pre-functionalized with low-molecular-weight azido groups grafted onto a hydrophilic adhesion promoter on a gold support. Ligands containing a compatible Click partner—such as dibenzocyclooctyne (DBCO)—can be covalently attached in a fast and selective manner via strain-promoted azide-alkyne cycloaddition (Click reaction).
Unlike classical EDC/NHS chemistry, DBCO/azide coupling is not time-critical; both reaction partners remain stable for weeks across pH 4—10. Their high selectivity minimizes side reactions and eliminates the need for quenching steps1. Consequently, immobilization via Click coupling is reliable, convenient, and flexible. Owing to rapid reaction kinetics, coupling under physiological conditions is feasible, although maximum immobilization densities may be somewhat lower; thus, electrostatic preconcentration remains advantageous for achieving high ligand loading.
The AZ sensor chip portfolio spans electrostatic immobilization capacities from approximately 6000 μRIU (AZD50L) to 40,000 μRIU (AZHC1500M), covering analytes from large viruses to small organic fragments. The surface-bound azido groups are highly hydrophilic and possess a strong dipole moment, further enhancing the overall bioinertness of the coating.
Key features:
- Versatile ligand coupling: Highly efficient and selective Click reaction enables covalent attachment of DBCO-functionalized ligands. Unlike classic amine coupling, ligand density can be iteratively increased after initial coupling, allowing fast optimization of immobilization levels and efficient sensor chip use.
- Superior efficiency: The high efficiency of the Click reaction permits ligand immobilization even under physiological conditions.
- DBCO functionalization: Ligands can be conveniently DBCO-modified using standard labeling protocols similar to biotinylation; site-specific conjugation via sortase-mediated ligation is also supported.
- Wide immobilization range: From several thousand to ~40,000 μRIU, suitable for analytes from whole cells and viruses to fragments < 300 Da.
- Bioinert nanoarchitecture: Combining a hydrophilic adhesion promoter, hydrated polycarboxylate matrix (CMD or HC), and surface azido groups minimizes nonspecific binding.
- Application versatility: Suitable for kinetic, equilibrium, and concentration analyses, as well as diverse screening applications in drug discovery.
| Product code 3 | AZD200M | AZHC30M | AZHC200M | AZHC1500M |
|---|---|---|---|---|
| Base coating | 3D, 200 nm bioinert CM-dextran (medium density) | 3D, 30 nm bioinert polycarboxylate (medium density) | 3D, 200 nm bioinert polycarboxylate (medium density) | 3D, 1500 nm bioinert polycarboxylate (medium density) |
| Covalent immobilization capacity [µRIU] 4 | ≈ 13,000 (30,000) | ≈ 10,000 (18,000) | ≈ 13,000 (26,000) | ≈ 20,000 (40,000) |
| Recommended ligands |
|
|||
| Recommended analytes |
|
|
|
|
| Intended purpose |
|
|
|
|
1 This is valid only when the analyte lacks its own Click-reactive functionality; otherwise, unintended covalent attachment may occur.
2 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
3 Table includes a selection from XanTec’s full AZ sensor chip portfolio.
4 Covalent immobilization capacity was assessed by injecting 100 µg/mL DBCO-modified streptavidin in 5 mM sodium acetate buffer (pH 5.0), with 1 µRIU corresponding approximately to 1 RU. Electrostatic preconcentration capacity is shown in brackets.
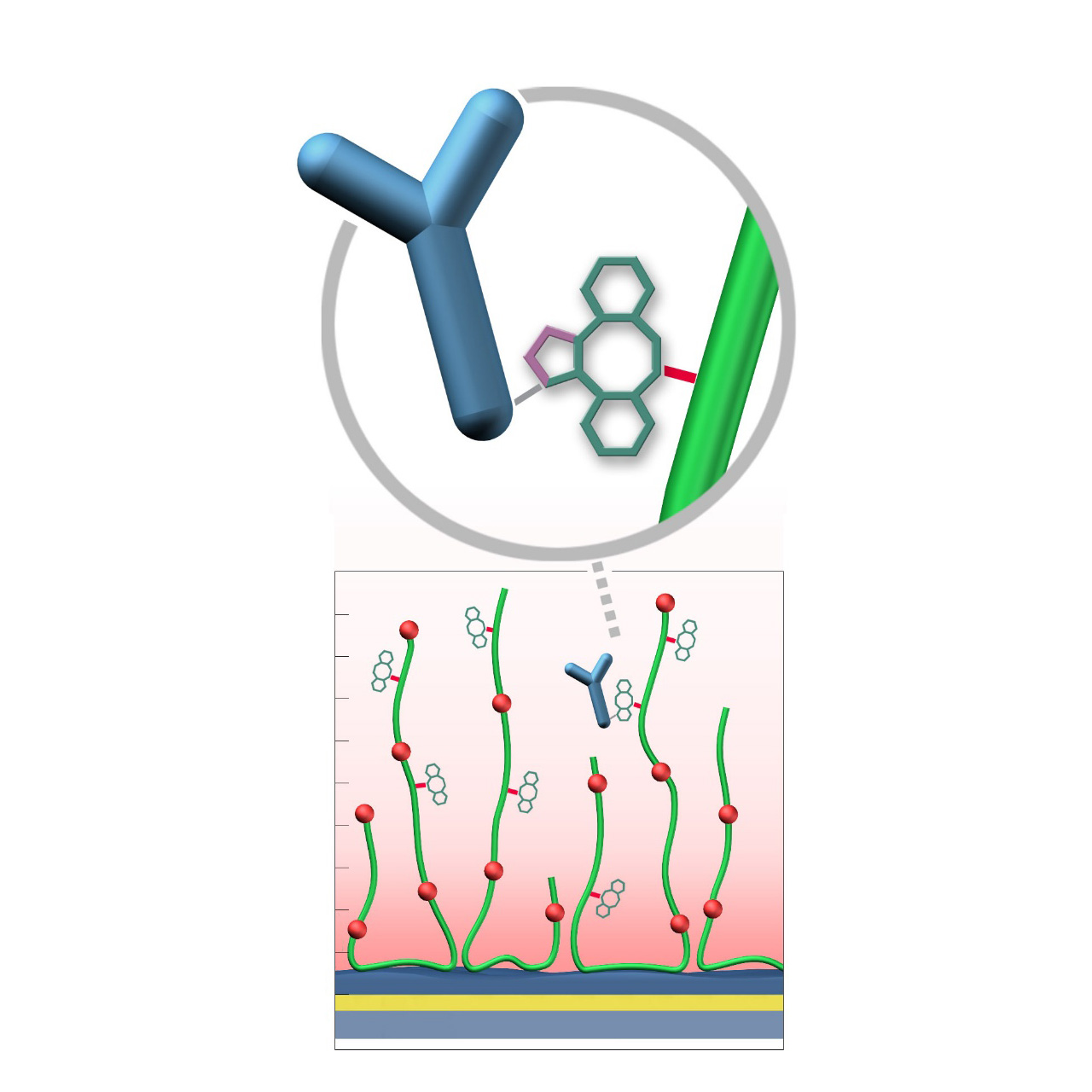
DBCO–modified sensor chips
XanTec’s DBCO sensor chips (DC) are coated with a dibenzocyclooctyne (DBCO)-derivatized bioinert carboxymethyl-dextran (CMD) matrix grafted onto a hydrophilic adhesion promoter on a gold support. Ligands containing a compatible Click partner—such as an azide group—can be covalently attached rapidly and selectively via strain-promoted azide-alkyne cycloaddition (SPAAC, Click reaction).
Unlike classical EDC/NHS amine-coupling, DBCO/azide Click chemistry is not time-critical; both reaction partners remain stable for weeks across pH 4-10. Their high selectivity minimizes side reactions and eliminates the need for quenching steps1. Consequently, Click-based immobilization is reliable, convenient, and operationally flexible. Due to the fast reaction kinetics, coupling under physiological conditions is feasible; however, maximum immobilization densities may be somewhat lower. Therefore, electrostatic preconcentration remains advantageous for achieving high ligand loading.
DBCO-modified surfaces exhibit a higher hydrophobicity than azide-modified counterparts or unmodified CMD coatings. This can lead to increased nonspecific binding, which is at least partially mitigated by the presence of nonionic detergents (e.g., Tween) in the running buffer. DBCO-modified sensor chips are most useful when the ligand naturally contains azide functionalities. Otherwise, the azide-modified sensor coating combined with a DBCO-modified ligand is generally preferred due to its higher intrinsic bioinertness.
Key features:
- Versatile ligand coupling: Efficient and selective Click reaction enables covalent attachment of azide-functionalized ligands. Unlike classic amine coupling, ligand density can be iteratively increased after initial coupling, allowing fast optimization of immobilization levels and efficient sensor chip use.
- Superior efficiency: High efficiency of the Click reaction permits ligand immobilization even under physiological conditions.
- Azide functionalization: Ligands can be conveniently azide-modified using labeling strategies analogous to biotinylation; site-specific conjugation via azide-containing unnatural amino acids is also feasible.
- Application versatility: Suitable for kinetic, equilibrium, and concentration analyses, as well as diverse screening applications in drug discovery.
| Product code | DCD50L | DCD200M |
|---|---|---|
| Base coating | 3D, 30 nm bioinert CM-dextran (low density) | 3D, 200 nm bioinert polycarboxylate (medium density) |
| Covalent immobilization capacity [µRIU] 2 | ≈ 3,000 | ≈ 11,000 |
| Recommended ligands |
|
|
| Recommended analytes |
|
|
| Intended purpose |
|
|
1 This is valid only when the analyte lacks its own Click-reactive functionality; otherwise, unintended covalent attachment may occur.
2 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
3Covalent immobilization capacity was assessed by injecting 100 µg/mL azide-modified bovine serum albumin (BSA) in 5 mM sodium acetate buffer (pH 5.0), with 1 µRIU corresponding approximately to 1 RU.
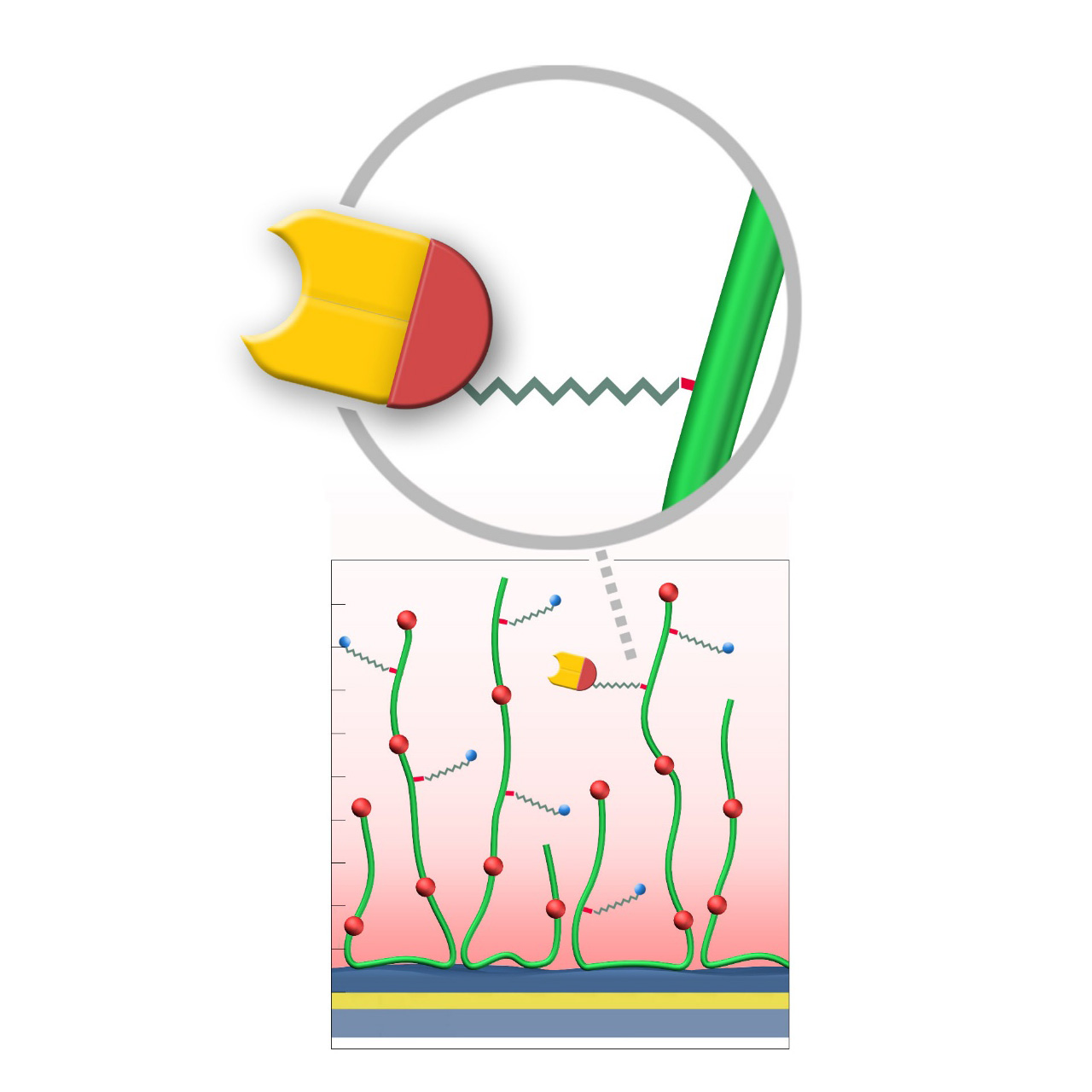
Chloroalkane–modified sensor chips
XanTec’s HOP, HOHC, and HOD sensor chips are coated with either a bioinert 2D carboxylate (HOP) or a 3D polycarboxylate hydrogel (HOHC, HOD), each derivatized with short-chain chloroalkanes. These functional groups form stable covalent bonds with fusion proteins bearing a 33 kDa Halo tag. Reaction is rapid, highly specific, and occurs spontaneously under preconcentration conditions, ensuring uniform ligand orientation on the sensor surface.
Compared to conventional non-selective methods such as EDC/NHS activation, the Halo tag immobilization process offers substantial advantages. It is not only significantly more convenient, but also allows for a more controlled and well-defined immobilization. By avoiding random ligand orientation and ligand crosslinking, which often occur with EDC/NHS coupling, this approach results in a homogeneous ligand population, ultimately leading to improved data quality and reproducibility in kinetic and affinity assays. Consequently, experimental workflows are streamlined, requiring fewer optimization steps, while the consistency and integrity of kinetic interaction analyses are enhanced, making it easier to draw reliable conclusions about binding kinetics and affinities.
Adequate electrostatic preconcentration remains essential for successful protein immobilization. However, traditional preconcentration scouting cannot be performed because Halo-tagged fusion proteins would directly bind to the sensor chip. Therefore, preconcentration and immobilization must be evaluated simultaneously or on a separate non-derivatized sensor chip. For further details, please refer to the product manual.
Key features:
- Faster assay development: No chemical activation required for covalent coupling. Ligand immobilization occurs directly under preconcentration conditions with adjustable ligand density, enabling rapid and reproducible assay setup.
- Exceptional stability: Covalent coupling of Halo-tagged protein results in drift-free, permanently immobilized ligands.
- Oriented immobilization: Site-directed attachment ensures maximum ligand activity.
- Versatile capture capacity: Available on CMDP, CMD, and HC base coatings, offering different immobilization densities for specific experimental requirements.
| Product code | HOP | HOD200M | HOHC200M |
|---|---|---|---|
| Base coating | 2D, ultra-short CM-dextran (high density) |
3D, 200 nm bioinert CM-dextran (medium density) |
3D, 200 nm bioinert polycarboxylate (medium density) |
| Immobilization capacity [µRIU] 2 | ≈ 3,000 | ≈12,000 | ≈15,000 |
| Recommended ligands | Halo-tagged peptides and proteins | ||
| Recommended analytes |
|
|
|
| Intended purpose |
|
|
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 Based on specific binding of 30 µg/mL HaloTag®-GST fusion protein (61 kDa) in 5 mM sodium acetate pH 5.0, with 1 µRIU corresponding approximately to 1 RU.
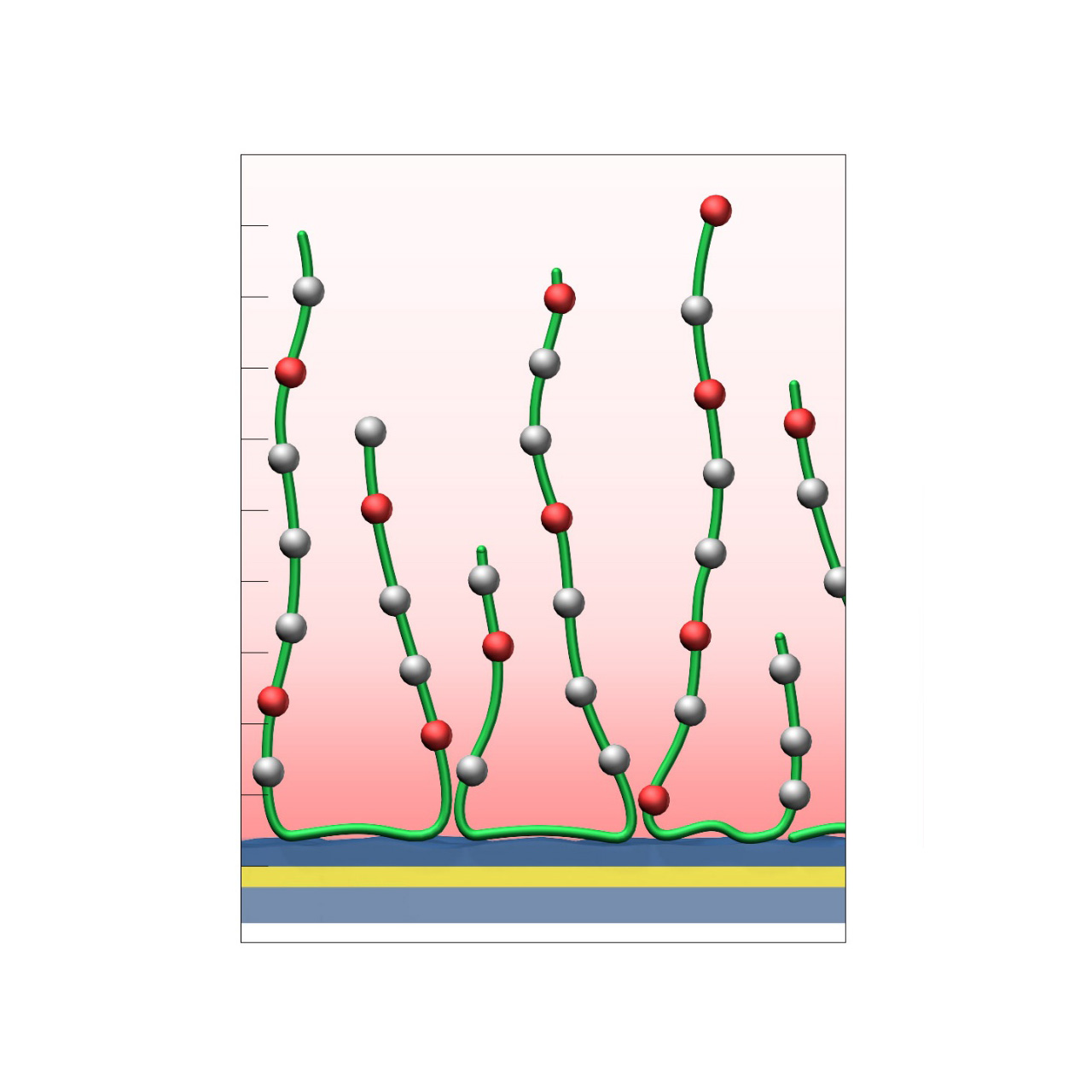
HLC–modified sensor chips
XanTec’s HLC sensor chips are based on a 3D hydrogel matrix composed of highly flexible, bioinert polycarboxylate chains grafted onto a hydrophilic adhesion promoter on a gold support. Ligands can be covalently attached through their amine, thiol, or aldehyde groups using established coupling chemistries such as EDC/NHS activation, thiol-maleimide coupling, or reductive amination. This versatility enables the immobilization of a wide range of biomolecules, including proteins, antibodies, peptides, nucleic acids, carbohydrates, and small organic compounds.
Compared with native HC coatings, a large fraction of carboxyl groups in the HLC polymer is converted to hydroxyl groups, substantially lowering the surface charge. As a result, HLC sensor chips are even more bioinert than their HC counterparts, and are particularly advantageous in experiments affected by persistent nonspecific binding—an issue often encountered with strongly positively charged biomolecules. The remaining carboxyl groups are typically sufficient to support electrostatic preconcentration and efficient covalent ligand coupling.
Key features:
- Reduced surface charge: Significantly lower nonspecific binding than native HC, especially with highly positively charged biomolecules; particularly suited for complex matrices such as cell culture media.
- Versatile ligand coupling: Covalent coupling through amine, thiol, or aldehyde groups via standard chemistries (EDC/NHS, maleimide, reductive amination).
- Wide immobilization range: From several thousand to ~18,000 μRIU, suitable for analytes from whole cells and viruses to fragments < 300 Da.
- Application versatility: Suitable for kinetic, equilibrium, and concentration analyses, as well as diverse screening applications in drug discovery.
- High chemical stability: Withstands typical regeneration conditions, maintaining consistent response levels and kinetic behavior after multiple regeneration cycles.
- No polysaccharide backbone: Lack of carbohydrate motifs prevents unwanted interactions with lectins or carbohydrate-binding proteins, making HLC sensor chips ideal for analyzing this class of biomolecules.
| Product code | HLC30M | HLC200M | HLC1500M |
|---|---|---|---|
| Base coating | 3D, 30 nm bioinert polycarboxylate (medium density, reduced charge) | 3D, 200 nm bioinert polycarboxylate (medium density, reduced charge) | 3D, 1500 nm bioinert polycarboxylate (medium density, reduced charge) |
| Immobilization capacity [µRIU] 2 | ≈ 6,000 | ≈ 11,000 | ≈ 18,000 |
| Recommended ligands |
|
||
| Recommended analytes |
|
|
|
| Intended purpose |
|
|
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
1 Preconcentration capacity determined by injecting 100 µg/mL bovine serum albumin (BSA) in 5 mM sodium acetate pH 5.0, with 1 µRIU corresponding approximately to 1 RU. Maximum covalent coupling yields can vary and depend strongly on the properties of the protein to be immobilized. Under optimal conditions, typical coupling efficiencies range from approximately 20–45% of the respective electrostatic preconcentration capacity, with acidic proteins generally exhibiting lower coupling efficiencies.
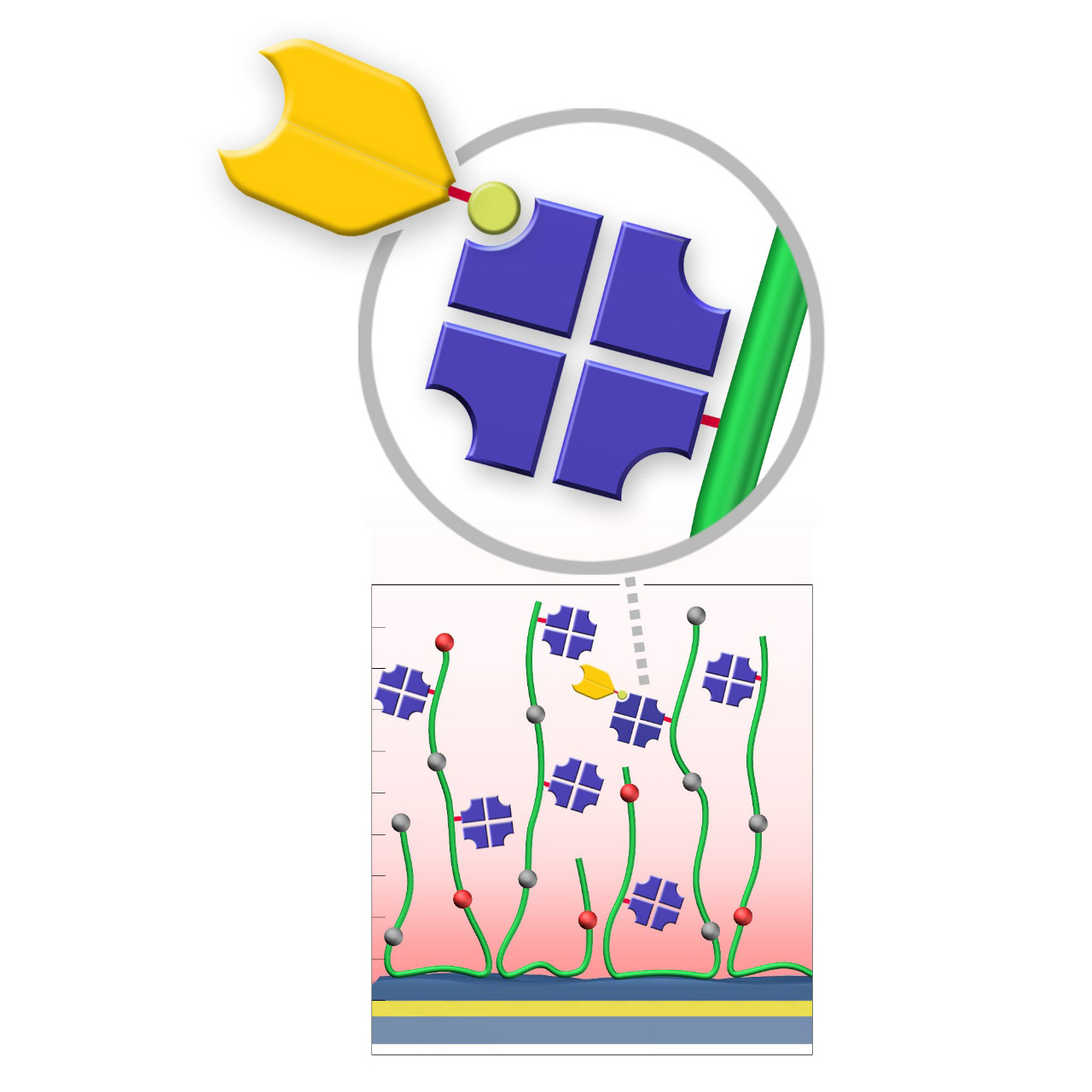
HCX sensor chips (NHS–preactivated)
XanTec’s HCX sensor chips are based on a 3D hydrogel matrix composed of flexible polycarboxylate chains grafted onto a hydrophilic adhesion promoter on a gold support. The carboxylate groups are partially pre-activated with NHS esters, providing medium to high activation level and enabling high immobilization capacities. This pre-activation significantly simplifies and accelerates ligand coupling, making HCX chips a powerful tool for streamlined workflows. They are particularly advantageous for spot immobilization in imaging SPR systems.
Although a certain fraction of a spotted ligand will immobilize when drying spots, electrostatic preconcentration substantially increases immobilization yields. A preconcentration scouting step is therefore recommended prior to coupling. However, small molecules freely diffuse into the hydrogel and do not require preconcentration for efficient immobilization.
Key features:
- Save time: Pre-activated sensor chips allow direct and convenient ligand immobilization, saving time and effort.
- Reliable results: Avoid variability of reagent quality and instead achieve consistent, reproducible immobilization every time.
- Flexibility: From moderate-capacity chips for protein-protein interaction studies to ultra-high-capacity formats for fragment screening, HCX covers the entire spectrum of ligand density requirements.
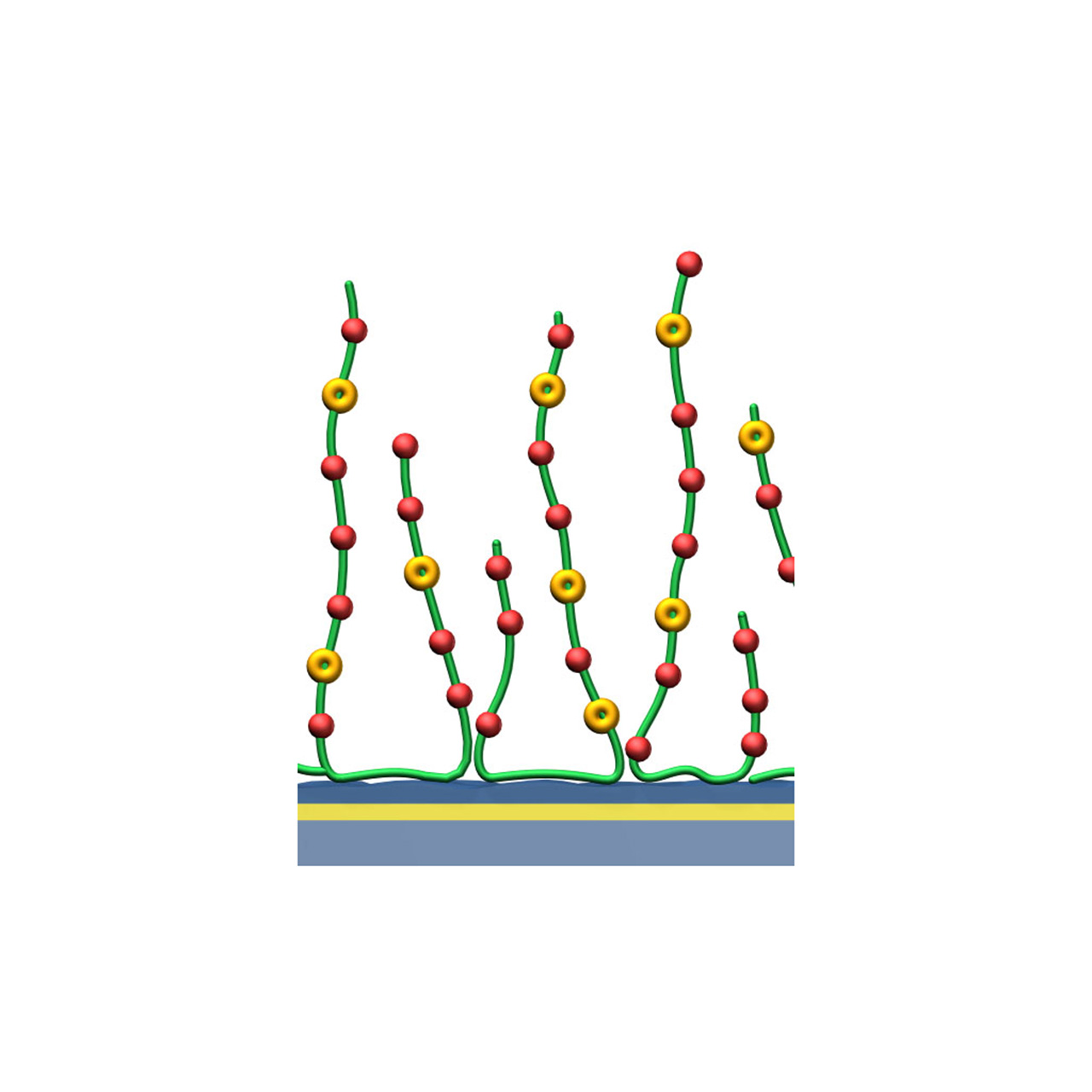
| Product code 2 | HCX30M | HCX200M | HCX1000M |
|---|---|---|---|
| Base coating | 3D, 30 nm bioinert polycarboxylate (medium density) NHS pre-activated |
3D, 200 nm bioinert polycarboxylate (medium density) NHS pre-activated |
3D, 1000 nm bioinert polycarboxylate (medium density) NHS pre-activated |
| Immobilization capacity [µRIU] 3 | ≈ 9,000 | ≈ 15,000 | ≈ 20,000 |
| Recommended ligands 4 |
|
|
|
| Recommended analytes |
|
|
|
| Intended purpose |
|
|
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 This overview represents a selection of the full HCX sensor chip portfolio.
3 Covalent immobilization capacity was assessed by injecting 100 µg/mL bovine serum albumin (BSA) in 5 mM sodium acetate pH 5.0, with 1 µRIU corresponding approximately to 1 RU.
4 Ligands require an NHS-reactive amine.
Biotin–modified sensor chips
XanTec's biotin-modified sensor chips are coated with a bioinert (poly)carboxylate matrix (CMD or HC), pre-functionalized with a low-molecular-weight biocytin group. Owing to the exceptionally high affinity of biotin for avidin family proteins (dissociation constant ≈ 10⁻¹⁵ M for streptavidin), these chips enable highly stable immobilization with essentially negligible dissociation of bound streptavidin or streptavidin-ligand complexes.
XanTec's biotin sensor chips have gained popularity due to their compatibility with Switchavidin, an avidin derivative with a switchable affinity for biotin. This allows quantitative ligand removal from the biotin-functionalized surface, which is difficult and time-consuming when using streptavidin. In typical workflows, Switchavidin is pre-incubated with a biotinylated ligand at a controlled stoichiometry, followed by immobilization of the Switchavidin-ligand complex on the biotin sensor surface. Without this precomplex formation, Switchavidin can become buried within the hydrogel matrix, significantly reducing the effective ligand-binding capacity.
Key features:
- Regenerable with Switchavidin: Enables reversible capture of biotinylated ligands via controlled binding and removal of Switchavidin.
- Oriented immobilization: Precise biotinylation strategies (e.g., AviTag) support uniform ligand orientation, maximizing activity and reproducibility.
- Versatile capture capacity: Available on CMD and HC base coatings, offering different capture capacities and sensor matrices to match specific experimental needs.
- High chemical stability: Supports stringent regeneration conditions for the effective removal of Switchavidin, and potentially also streptavidin, from the sensor chip.
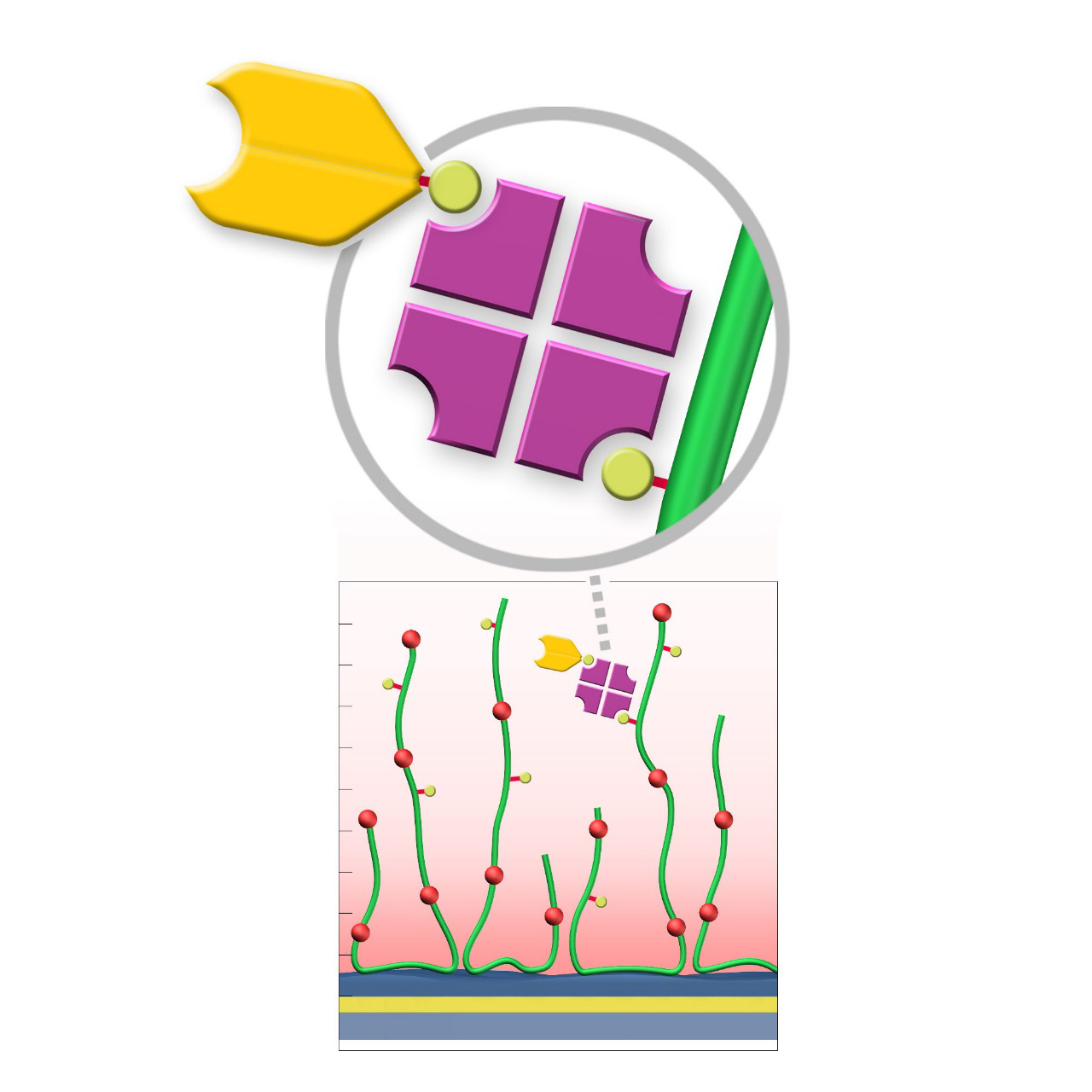
| Product code | BP | BD200M | BHC200M |
|---|---|---|---|
| Base coating | 2D, ultra-short CM-dextran (high density) |
3D, 200 nm bioinert CM-dextran (medium density) |
3D, 200 nm bioinert polycarboxylate (medium density) |
| Immobilization capacity [µRIU] 2 | ≈ 800 | ≈ 7,000 | ≈ 7,000 |
| Recommended ligands | biotinylated
|
biotinylated
|
|
| Recommended analytes |
|
|
|
| Intended purpose |
|
|
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 Based on specific binding of 100 µg/mL streptavidin in phosphate-buffered saline (PBS), with 1 µRIU corresponding approximately to 1 RU.
Sensor chips with lipophilic anchors (HPP, LP and LD)
XanTec’s LP and LD sensor chips are coated with a bioinert 2D (CMDP) or 3D (CMD500L) carboxymethyl-dextran polymer matrix, partially functionalized with lipophilic alkyl anchor groups. In contrast to solely hydrophobic 2D chip surfaces such as HPP, LP and LD sensor chips are partially hydrophilic and conserve the functionality of lipid bilayer structures.
HPP sensor chips provide a dense 2D hydrophobic coating, which allows formation of stabilized lipid monolayers. The hydrophobic surface enables lipid monolayer adsorption for studying lipid-associated interactions.
LP sensor chips consist of a hydrophilic 2D CMDP cushion functionalized with lipophilic alkyl anchors. This architecture enables reversible adsorption and rupture of lipid vesicles, resulting in the formation of a supported lipid bilayer (SLB). Membrane proteins incorporated within vesicles typically remain structurally intact and maintain biological activity after bilayer formation, making LP chips suitable for interaction studies involving moderate- to high-molecular-weight analytes, such as proteins. LP surfaces also serve as robust supports for SLB model systems, offering multiple regeneration cycles.
LD sensor chips are coated with a hydrophilic, 3D hydrogel based on brush-structured carboxymethyl-dextran. Lipid vesicles can diffuse into the LD hydrogel matrix. Incorporation of the lipophilic anchor groups allows the reversible capture of such vesicles. The shape of the vesicles usually remains intact, making them suitable for biomolecular investigation of (trans)membrane proteins such as G protein-coupled receptors (GPCRs). Compared to LP, the immobilization capacity of LD sensor chips is ≈ 50% higher, allowing investigation of biomolecular interactions, including smaller analytes.
Key features:
- HPP – Hydrophobic coating: Enables formation of a supported lipid monolayer; user-prepared liposomes adsorb and reorganize spontaneously into a monolayer with hydrophilic headgroups facing the bulk solution; not suitable for transmembrane proteins (risk of denaturation upon vesicle adsorption).
- LP – Supported lipid bilayer (SLB): Hydrophilic 2D coating with hydrophobic anchors supports reversible adsorption/rupture of vesicles and SLB formation; stabilization and preservation of (trans)membrane proteins for interaction analysis; regenerable surface suitable for multiple assay cycles.
- LD – Vesicle immobilization in 3D hydrogel: Hydrophobic anchors enable reversible immobilization of intact lipid vesicles and associated (trans)membrane proteins (e.g., GPCRs); higher immobilization densities support investigation of small analytes; regenerable surface enables multiple assay cycles.
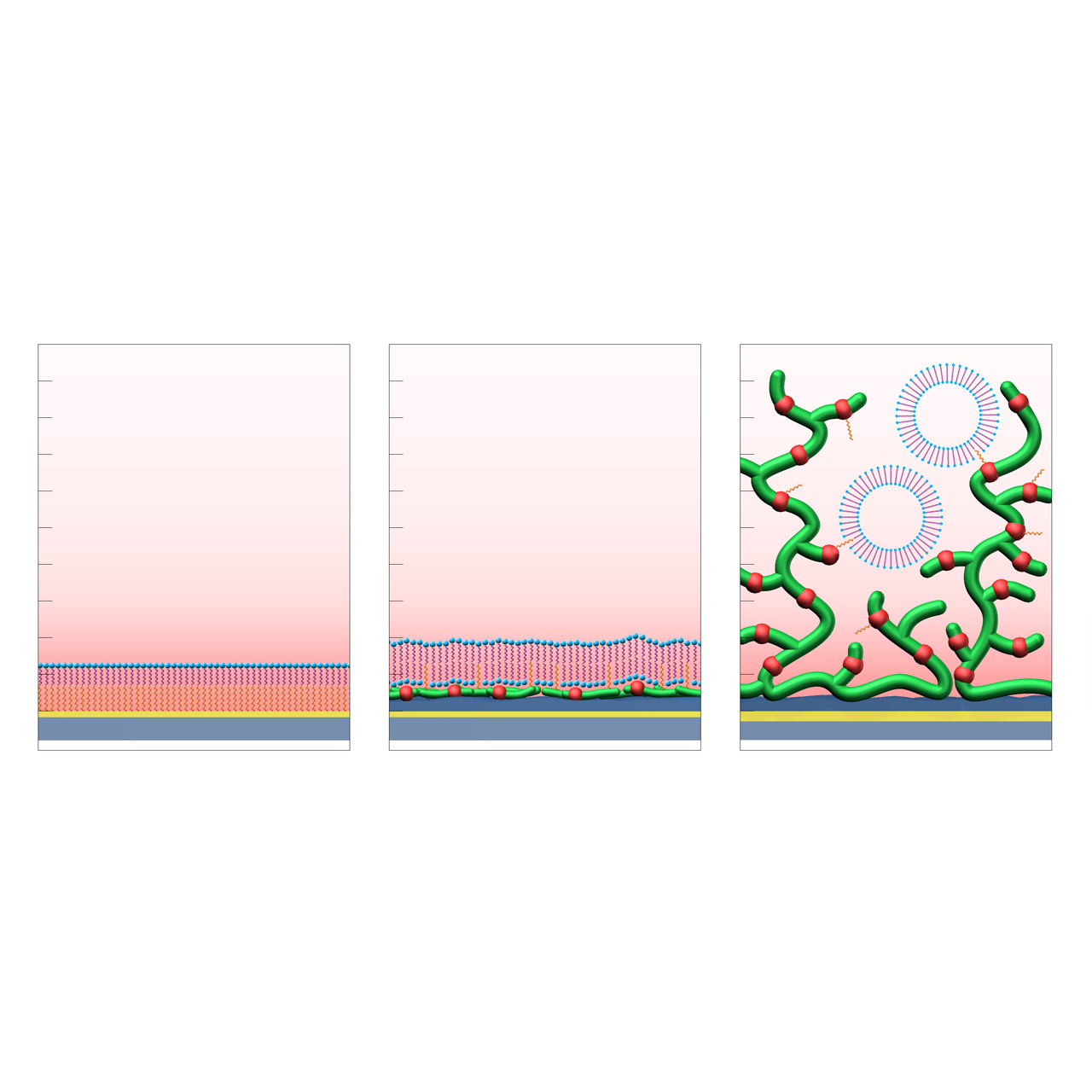
HPP (left): formation of a supported lipid monolayer (hydrophilic headgroups in blue, aliphatic tails in purple) on a hydrophobic planar self-assembled alkyl monolayer (SAM, orange).
LP (center): formation of a supported lipid bilayer on a hydrophilic CMDP base coating functionalized with hydrophobic anchor groups (orange).
LD (right): immobilization and stabilization of intact lipid vesicles within a 3D CMD sensor matrix modified with hydrophobic anchor groups (orange).1
| Product code | HPP | LP | LD |
|---|---|---|---|
| Base coating | 2D, hydrophobic planar alkyl layer | 2D, carboxymethyl-dextran surface, partially alkyl-derivatized |
3D, carboxymethyl-dextran surface, partially alkyl-derivatized |
| Immobilization capacity [µRIU]2 | n. a. | ≈ 9,000 | ≈ 13,000 |
| Recommended ligands |
|
|
|
| Recommended analytes |
|
|
|
| Intended purpose |
|
|
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 Capacity determined by injecting an in-house vesicle formulation, with 1 µRIU corresponding approximately to 1 RU.
Alginate–modified sensor chips
XanTec’s alginate (AL) sensor chips are based on a 50 nm alginate hydrogel matrix grafted onto a hydrophilic adhesion promoter on a gold support. The negatively charged carboxyl groups distributed along the polysaccharide chains enable efficient electrostatic preconcentration of ligands prior to covalent coupling.
Ligands can be covalently attached through their amine, thiol, or aldehyde groups using standard coupling chemistries such as EDC/NHS activation, thiol–maleimide conjugation, or reductive amination. This allows immobilization of proteins, antibodies, peptides, nucleic acids, and small molecules. Efficient protein immobilization requires electrostatic preconcentration before covalent attachment.
XanTec’s AL sensor chips were specifically developed as a functionally equivalent alternative to Bio-Rad’s ProteOn GLM sensor chips. They provide ProteOn users with a compatible alginate-based hydrogel surface and represent a reliable option when CMD- or HC-based coatings are unsuitable or cannot be applied.
In addition, AL sensor chips serve as benchmark coatings for assessing nonspecific binding during the development and evaluation of biomedical and antifouling surface chemistries.
Key features:
- Alternative to ProteOn GLM sensor chips: Provides a 3D alginate-based hydrogel matrix for covalent ligand immobilization.
- Versatile ligand coupling: Supports amine-, thiol-, and aldehyde-based coupling using established chemistries (EDC/NHS, maleimide, reductive amination).
- Benchmark surface: Suitable as a reference coating for evaluating nonspecific binding and new biomedical coating materials.
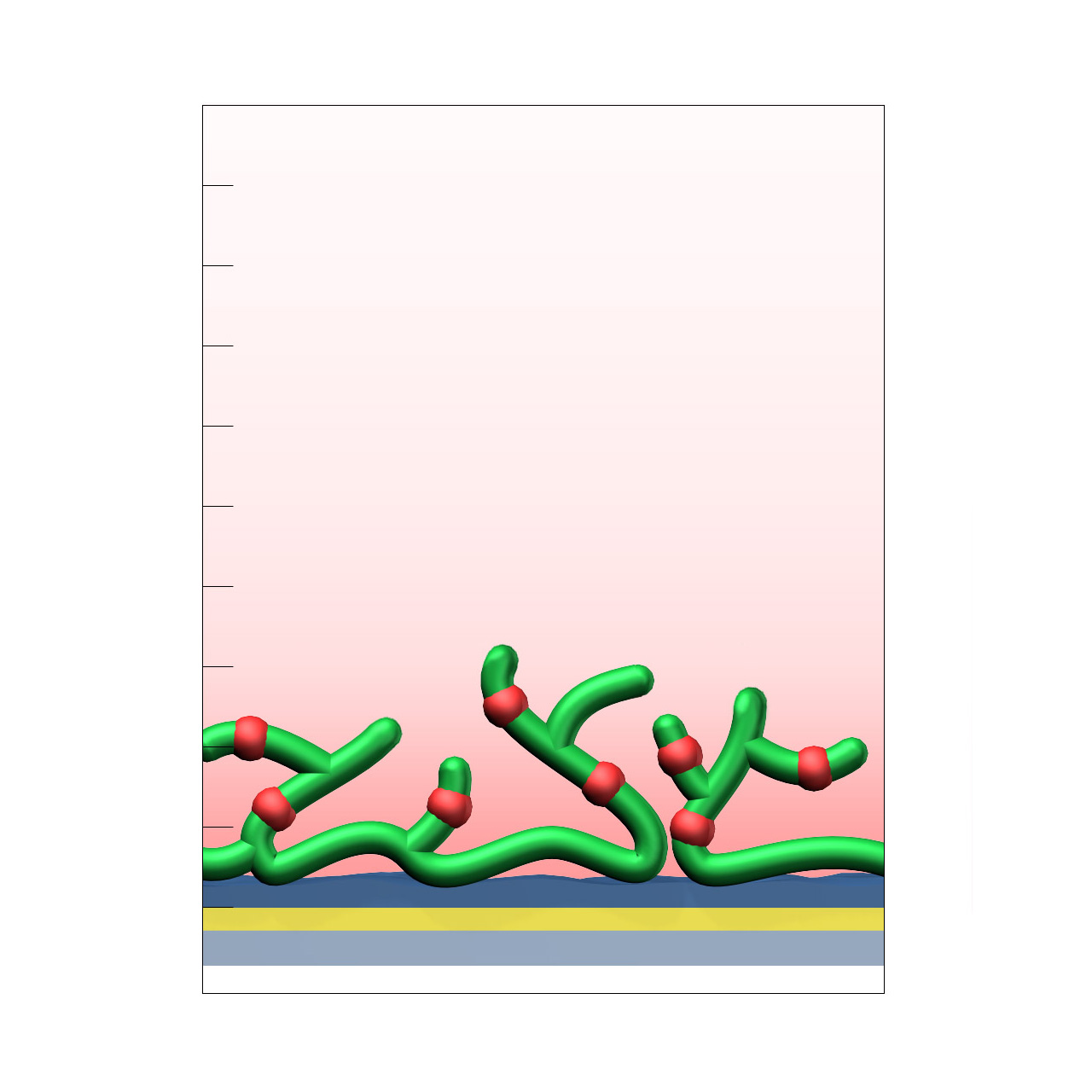
| Product code | AL |
|---|---|
| Base coating | 3D, 50 nm alginate (medium density) |
| Electrostatic preconcentration capacity [µRIU]2 | ≈ 13,000 |
| Recommended ligands |
|
| Recommended analytes |
|
| Intended purpose |
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 Preconcentration capacity determined by injecting 100 µg/mL BSA in 5 mM sodium acetate pH 5.0, with 1 µRIU corresponding approximately to 1 RU. Maximum covalent coupling yields depend on ligand properties and typically range from approximately 20–45% of the respective electrostatic preconcentration capacity.
C-type sensor chips
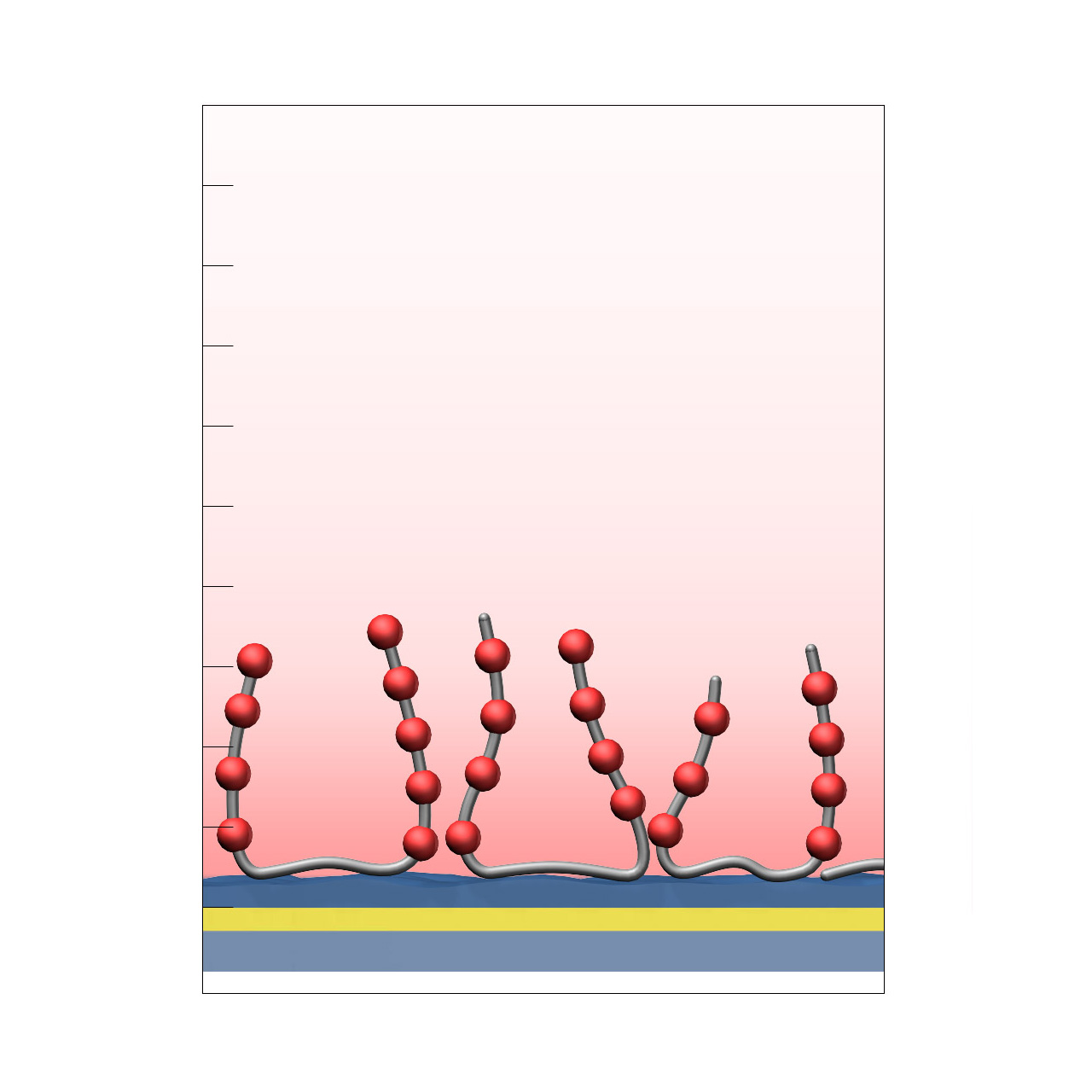
XanTec’s C-type sensor chips are based on a synthetic 3D hydrogel matrix composed of linear aliphatic polycarboxylate chains with very high charge density, grafted onto a hydrophilic adhesion promoter on a gold support. Ligands can be covalently attached via their amine, thiol, or aldehyde groups using established coupling chemistries such as EDC/NHS activation, thiol–maleimide conjugation, or reductive amination. This enables immobilization of proteins, antibodies, peptides, nucleic acids, and small molecules.
The exceptionally high charge density renders the surface susceptible to nonspecific binding of cationic biomolecules; however, the aliphatic polymer backbone provides outstanding chemical robustness. This makes C-type coatings particularly well suited for demanding chemical environments and for non-biological applications that require a dense and chemically well-defined carboxylate (COOH) surface, such as solid-phase syntheses or the preparation of metal–organic frameworks (MOFs).
Key features:
- Exceptional chemical stability: The aliphatic polymer backbone provides the highest chemical robustness within XanTec’s portfolio, enabling use under harsh chemical conditions and supporting non-biological applications requiring a dense COOH surface.
- Very high carboxyl group density: Enables highly efficient ligand immobilization under electrostatic preconcentration conditions.
- Versatile coating thickness: Available in thicknesses from 30 to 150 nm, allowing flexible adaptation to specific experimental requirements.
| Product code | C30M | C80M | C150D |
|---|---|---|---|
| Base coating | 3D, 30 nm aliphatic polycarboxylate (medium density) |
3D, 80 nm aliphatic polycarboxylate (medium density) |
3D, 150 nm aliphatic polycarboxylate (high density) |
| Electrostatic preconcentration capacity [µRIU]2 | ≈ 9,000 | ≈ 23,000 | ≈ 50,000 |
| Recommended ligands |
|
||
| Recommended analytes |
|
|
|
| Intended purpose |
|
||
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 Preconcentration capacity determined by injecting 100 µg/mL bovine serum albumin (BSA) in 5 mM sodium acetate pH 5.0, with 1 µRIU corresponding approximately to 1 RU. Maximum covalent coupling yields depend on ligand properties and typically range from approximately 25–45% of the respective electrostatic preconcentration capacity.
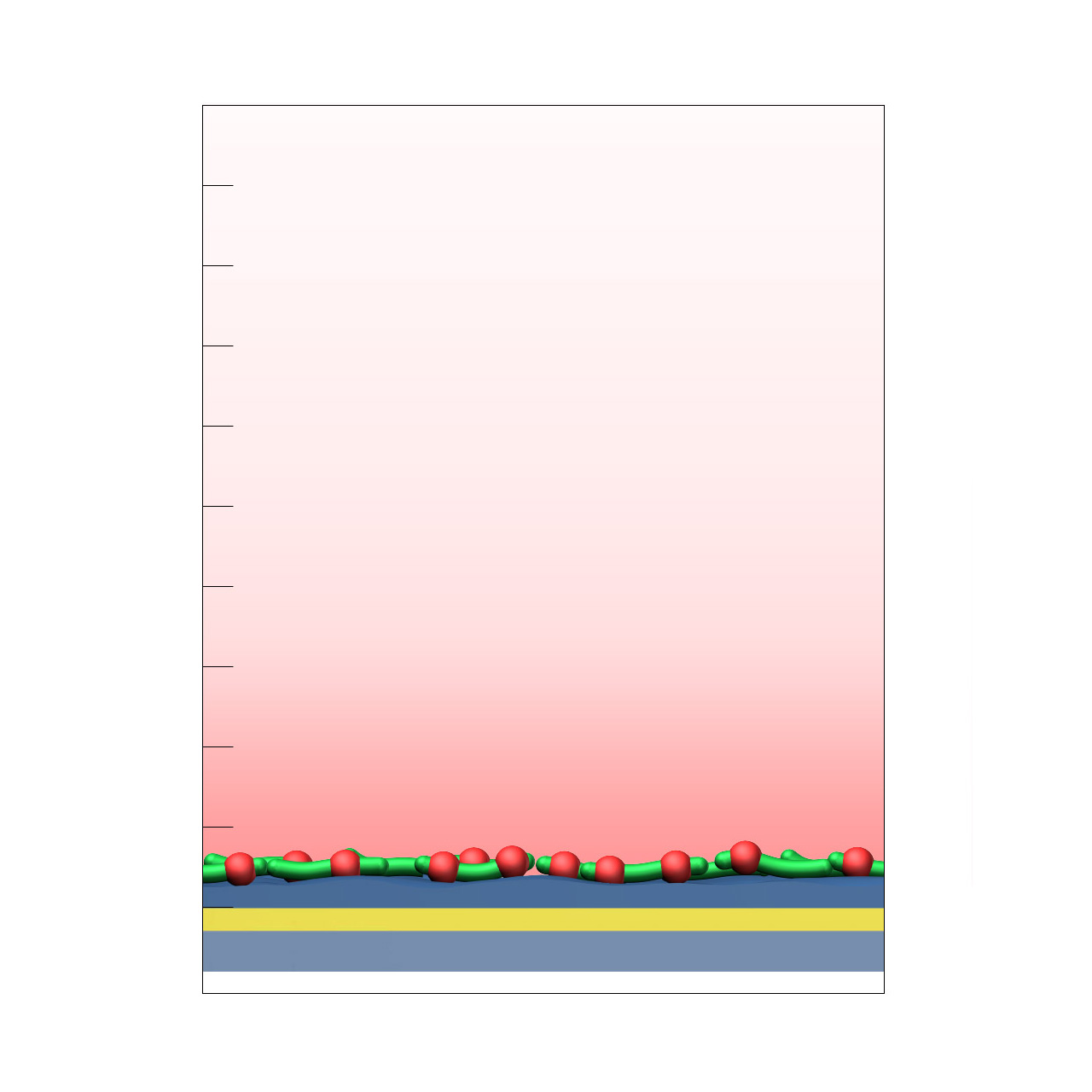
CMPG–modified sensor chips
XanTec’s CMPG sensor chips are based on a short, dendritic carboxymethylated polyglycerol (CMPG) 2D sensor matrix grafted onto a hydrophilic adhesion promoter on a gold support. Ligands can be covalently attached via their amine, thiol, or aldehyde groups using standard coupling chemistries such as EDC/NHS activation, thiol–maleimide conjugation, or reductive amination. This enables immobilization of proteins, antibodies, peptides, nucleic acids, and small molecules.
Although the protein immobilization capacity is relatively low (typically ≈ 1,000–2,000 µRIU), the highly hydrophilic, dendritic polymer architecture provides outstanding resistance to nonspecific binding. CMPG surfaces are therefore particularly well suited for experiments challenged by persistent nonspecific interactions, especially those involving strongly positively charged biomolecules or complex sample matrices such as cell culture media.
Key features:
- Very low background binding: Excellent suppression of nonspecific binding, particularly of highly cationic biomolecules; well suited for complex sample matrices such as cell culture media.
- Versatile ligand coupling: Supports covalent attachment through amine, thiol, or aldehyde groups using established chemistries (EDC/NHS, maleimide, reductive amination).
- Low sensor matrix profile: The planar 2D coating enables reliable kinetic analysis of systems with fast association and dissociation rates.
- No polysaccharide backbone: Absence of carbohydrate motifs prevents unwanted interactions with lectins or carbohydrate-binding proteins.
| Product code | CMPG |
|---|---|
| Base coating | 2D, carboxymethylated polyglycerol (high density) |
| Electrostatic preconcentration capacity [µRIU]2 | ≈ 2,000 |
| Recommended ligands |
|
| Recommended analytes |
|
| Intended purpose |
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 Preconcentration capacity determined by injecting 100 µg/mL BSA in 5 mM sodium acetate pH 5.0, with 1 µRIU corresponding approximately to 1 RU. Maximum covalent coupling yields depend strongly on ligand properties and typically range from approximately 20–45% of the respective electrostatic preconcentration capacity.
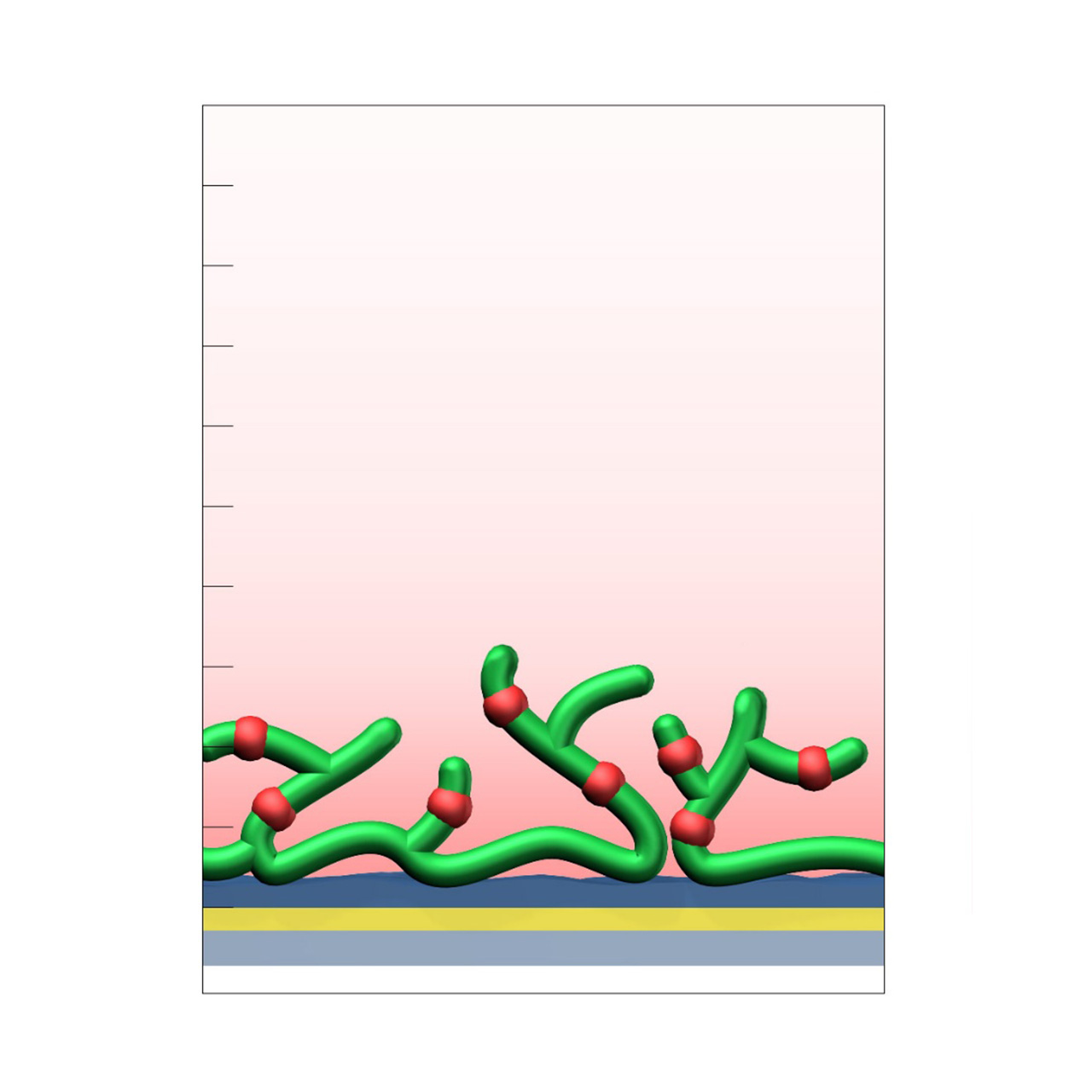
Heparin–modified sensor chips
XanTec’s heparin (HEP) sensor chips are based on a 50 nm heparin hydrogel matrix grafted onto a hydrophilic adhesion promoter on a gold support. Ligands can be covalently attached via their amine, thiol, or aldehyde groups using standard coupling chemistries such as EDC/NHS activation, thiol–maleimide conjugation, or reductive amination. Efficient protein immobilization requires electrostatic preconcentration prior to covalent coupling.
Although heparin surfaces are not typically the first choice for routine kinetic or affinity analyses, they provide a valuable alternative when CMD- or HC-based sensor chips are unsuitable. In addition, HEP sensor chips serve as benchmark coatings for assessing nonspecific binding during the development and evaluation of biomedical or antifouling surface chemistries, particularly in applications focused on hemocompatibility.
Key features:
- Alternative to CMD or HC surfaces: Provides a 3D hydrogel matrix for covalent ligand immobilization when CMD or HC coatings are unsuitable.
- Versatile ligand coupling: Supports covalent attachment through amine, thiol, or aldehyde groups using established chemistries (EDC/NHS, maleimide, reductive amination).
- Benchmark surface: Useful as a reference coating for evaluating nonspecific binding and assessing new biomedical coating materials.
| Product code | HEP |
|---|---|
| Base coating | 3D, 50 nm heparin (medium density) |
| Electrostatic preconcentration capacity [µRIU]2 | ≈ 11,000 |
| Recommended ligands |
|
| Recommended analytes |
|
| Intended purpose |
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 Preconcentration capacity determined by injecting 100 µg/mL BSA in 5 mM sodium acetate pH 5.0, with 1 µRIU corresponding approximately to 1 RU. Maximum covalent coupling yields depend strongly on ligand properties and typically range from approximately 20–45% of the respective electrostatic preconcentration capacity.
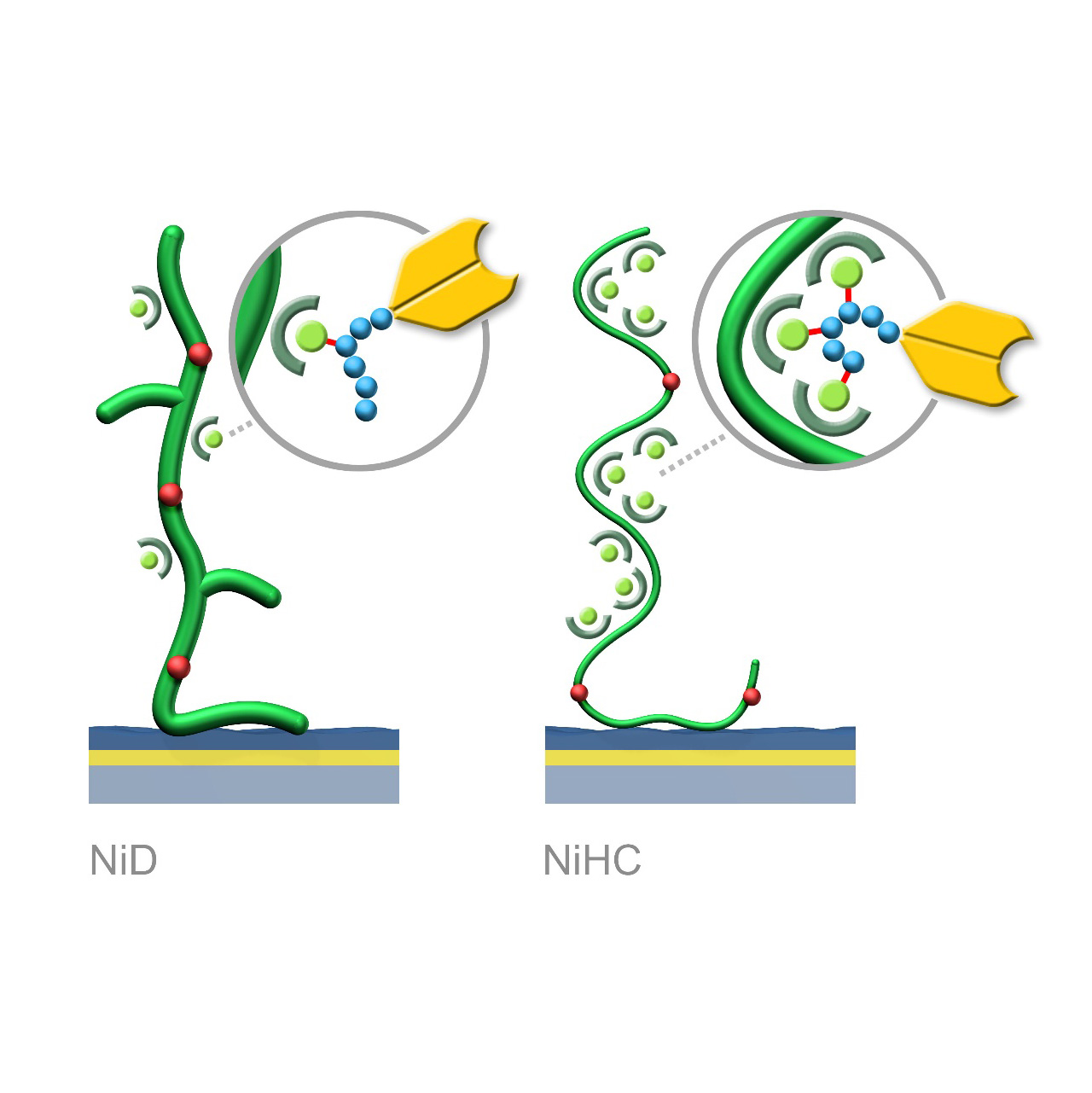
NTA–modified sensor chips (Ni)
XanTec’s NiD and NiHC sensor chips are coated with a bioinert (poly)carboxylate hydrogel matrix modified with nitrilotriacetic acid (NTA) functionalities. These sensor chips enable the reversible capture immobilization of His-tagged biomolecules through complex formation with transition metal ions, preferably Ni2+. For sufficient binding stability, the His-tag should contain 6–10 histidine residues. Complete regeneration of both NiD and NiHC surfaces is typically achieved by removal of chelated Ni2+ ions using chelating agents (e.g., EDTA) or competitive ligands such as imidazole.
Binding characteristics:
NiD sensor chips exhibit predominantly monovalent interactions between NTA(Ni2+) complexes and His-tags, resulting in dissociation rates (koff) in the range of 10−3 s−1.
NiHC sensor chips support multivalent binding due to the flexibility of the polymer chains, increasing binding stability by up to three orders of magnitude. Baselines after His-tagged ligand capture show minimal to no drift, with typical koff values of 10−5–10−6 s−1.1
A known limitation of NTA-based capture surfaces is the potential for nonspecific interactions between chelated Ni2+ ions and protein analytes or complex sample matrices (e.g., FBS). Proteins containing exposed histidines or metal-affinity motifs may bind non-selectively, increasing background signals. For assays sensitive to nonspecific binding, alternative immobilization strategies (e.g., anti-His-tag antibody capture) should be considered.
Key features:
- Fast assay development: No preconcentration or surface activation required; reversible ligand immobilization under physiological conditions.
- Easy regeneration: Chip surfaces regenerated using EDTA or imidazole injections.
- Outstanding stability (NiHC): Multivalent binding minimizes baseline drift during analysis.
- Oriented immobilization: His-tag–directed capture ensures maximum ligand activity.
- Versatile capture capacity: Available on CMD and HC base coatings for a broad range of analytes.
| Product code | NiP | NiD200M | NiHC200M | NiHC1500M |
|---|---|---|---|---|
| Base coating | 2D, ultra-short bioinert CM-dextran (high density) |
3D, 200 nm bioinert CM-dextran (medium density) |
3D, 200 nm bioinert polycarboxylate (medium density) |
3D, 1500 nm bioinert polycarboxylate (medium density) |
| Capture immobilization capacity [µRIU]4 | ≈ 120 | ≈ 400 | ≈ 1,200 | ≈ 2,000 |
| Ligands | His-tagged peptides and proteins | |||
| Recommended analytes |
|
|
|
|
| Intended purpose |
|
|
|
|
1 Maximum binding stability requires immobilization at approximately one-third of the chip’s maximum capture capacity.
2 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
3 Table includes a selection from XanTec’s full Ni sensor chip portfolio.
4 Binding capacities are based on the capture level of His6-peptide from a 5 µM solution after a 180 s stabilization period, with 1 µRIU corresponding approximately to 1 RU. Immobilization capacities of His-tagged proteins are considerably higher.
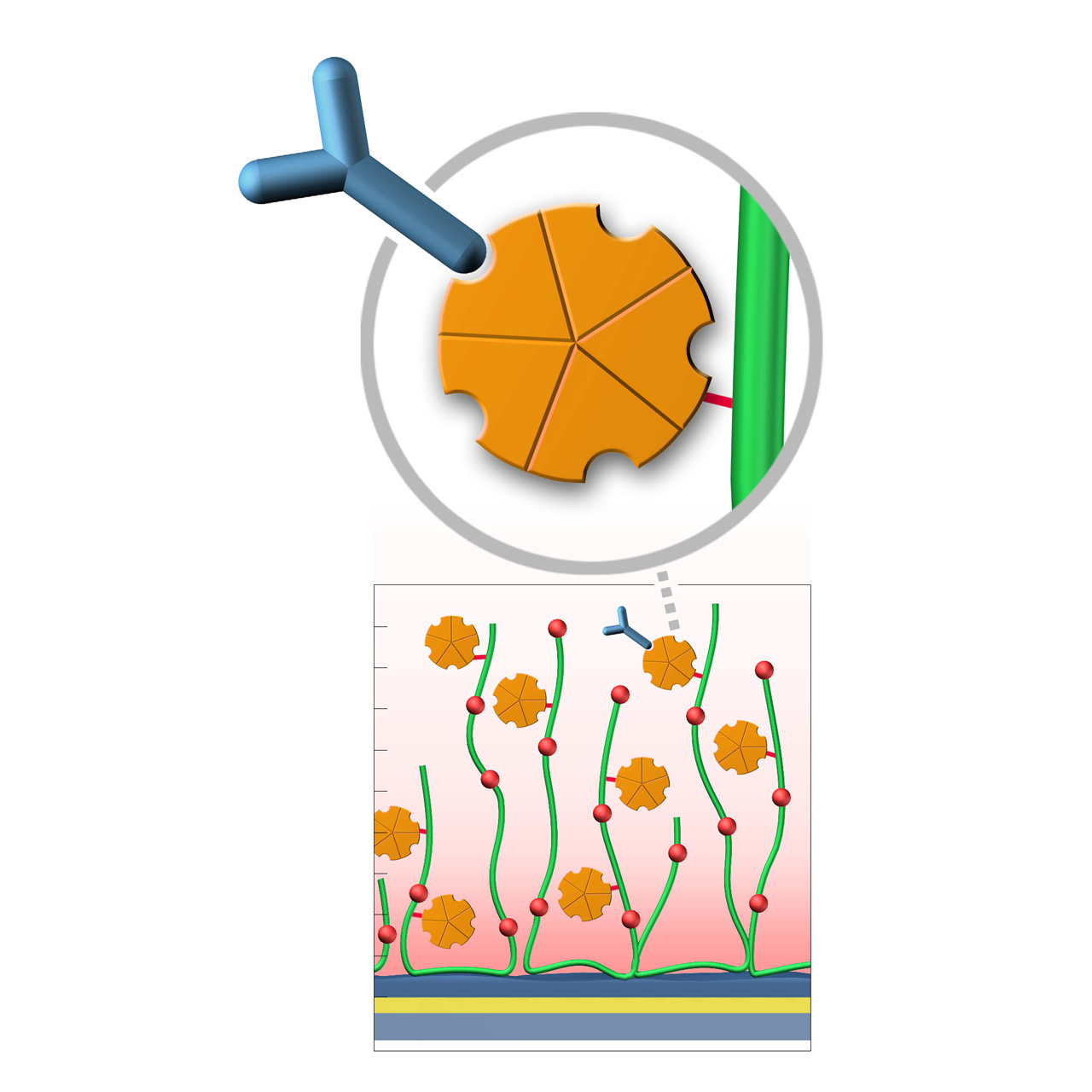
Protein A–modified sensor chips
XanTec’s Protein A (PA) sensor chips are coated with a bioinert polycarboxylate matrix pre-functionalized with a recombinant 47 kDa Protein A. This non-glycosylated variant contains five IgG-binding domains, providing high-affinity binding to the Fc region of all human IgG1, IgG2, and IgG4 subclasses. It also shows strong affinity for mouse IgG2a, IgG2b, and IgG3, as well as total IgG from cow, goat, sheep, rabbit, and other mammals.
Due to the slightly hydrophobic character of Protein A, some nonspecific binding may occur. The use of blocking agents (e.g., BSA) and/or nonionic detergents (e.g., Tween) in the running buffer is therefore recommended to minimize background signals. XanTec’s PA sensor chips are ready-to-use, eliminating time-intensive assay optimization and streamlining workflows.
Key features:
- Fast assay development: No preconcentration or surface activation required; reversible capture under physiological conditions.
- Simple regeneration: Regeneration using glycine·HCl pH 1.5–2.5.
- High stability: Robust capture surfaces enabling multiple reuse cycles.
- Oriented immobilization: Directed Fc-region binding ensures high ligand activity.
| Product code | PAP | PAD200L | PAHC30M | PAHC200M |
|---|---|---|---|---|
| Base coating | 2D, ultra-short bioinert CM-dextran (high density) |
3D, 200 nm bioinert CM-dextran (low density) |
3D, 30 nm bioinert polycarboxylate (medium density) |
3D, 200 nm bioinert polycarboxylate (medium density) |
| Capture immobilization capacity [µRIU]3 | ≈ 5,000 | ≈ 12,000 | ≈ 6,000 | ≈ 11,000 |
| Ligands | Fc region of human IgG1, IgG2, and IgG4; strong affinity for mouse IgG2a, IgG2b, IgG3, and total IgG from cow, goat, sheep, and rabbit | |||
| Recommended analytes |
|
|
|
|
| Intended purpose |
|
|
|
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 Table includes a selection from XanTec’s full PA sensor chip portfolio.
3 Maximum immobilization capacities are based on capture from a 100 µg/mL solution of Protein A-purified, pooled rabbit IgG in PBS, with 1 µRIU corresponding approximately to 1 RU.
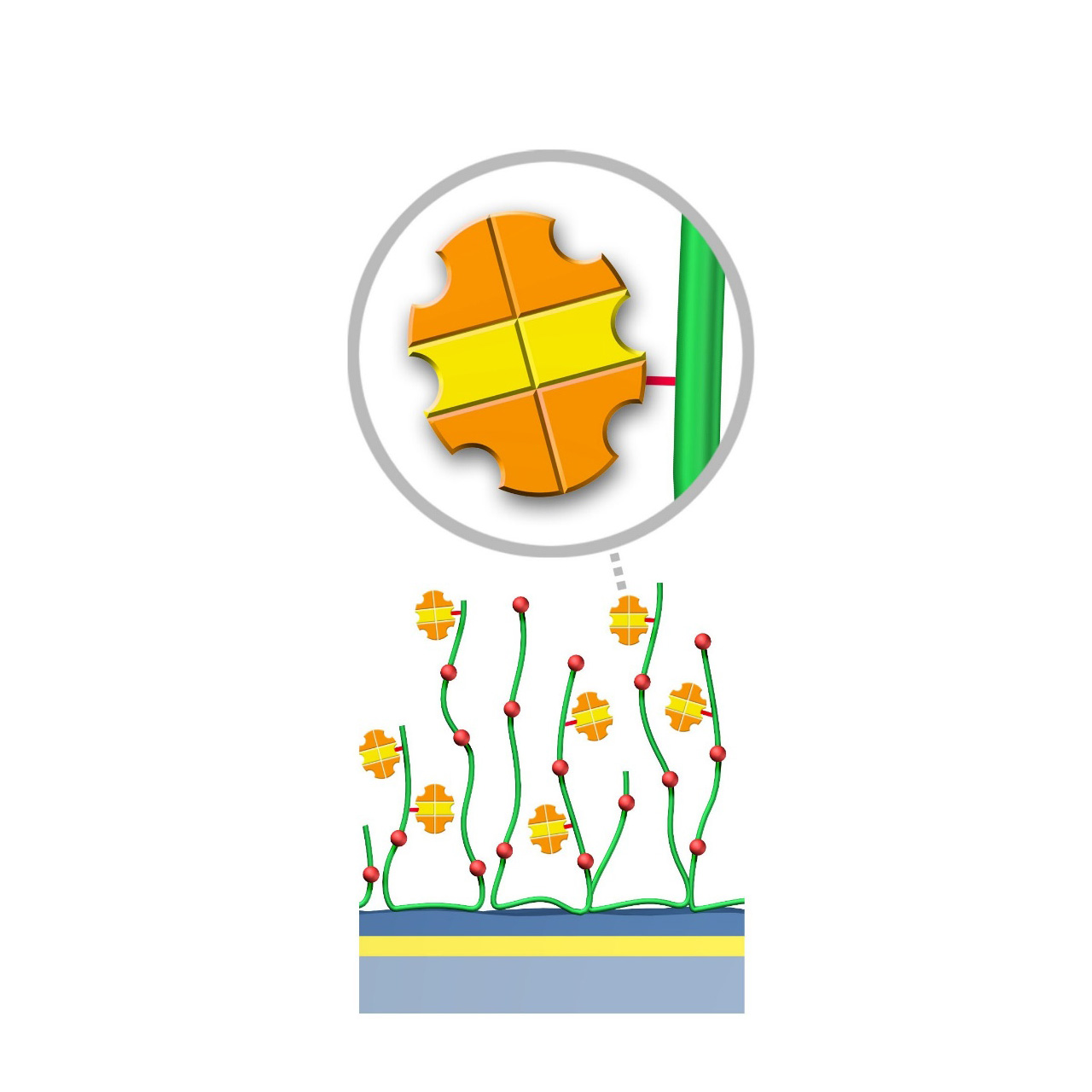
Protein AG–modified sensor chips
XanTec’s Protein AG (PAG) sensor chips are coated with a bioinert polycarboxylate matrix pre-functionalized with a recombinant 50.5 kDa Protein AG. This non-glycosylated variant combines four IgG-binding domains from Protein A and two IgG-binding domains from Protein G, providing high-affinity binding to the Fc region of all human IgG subclasses. In addition, it exhibits strong affinity for mouse IgG2a, IgG2b, and IgG3, as well as total IgG from cow, goat, sheep, rabbit, and other mammals.
To ensure highly specific IgG binding, non-essential domains (e.g., cell wall-, cell membrane-, and albumin-binding regions) have been removed from the recombinant Protein AG. Nevertheless, due to its slightly hydrophobic character, some nonspecific binding may occur. The inclusion of blocking agents (e.g., BSA) and/or nonionic detergents (e.g., Tween) in the running buffer is therefore recommended to minimize background signals. XanTec’s PAG sensor chips are ready-to-use, enabling rapid assay setup without time-intensive surface optimization.
Key features:
- Fast assay development: No preconcentration or surface activation required; controlled and reversible capture under physiological conditions.
- Broad antibody compatibility: Wider subclass and species coverage than Protein A.
- Simple regeneration: Regeneration using glycine·HCl pH 1.5.
- High stability: Robust capture surfaces support repeated regeneration cycles.
- Oriented immobilization: Directed Fc-region binding ensures high ligand activity.
| Product code | PAGP | PAGD200L | PAGHC30M | PAGHC200M |
|---|---|---|---|---|
| Base coating | 2D, ultra-short bioinert CM-dextran (high density) |
3D, 200 nm bioinert CM-dextran (low density) |
3D, 30 nm bioinert polycarboxylate (medium density) |
3D, 200 nm bioinert polycarboxylate (medium density) |
| Capture immobilization capacity [µRIU]3 | ≈ 5,000 | ≈ 12,000 | ≈ 6,000 | ≈ 11,000 |
| Ligands | Fc region of all human IgG subclasses; strong affinity for mouse IgG2a, IgG2b, IgG3, and total IgG from cow, goat, sheep, and rabbit | |||
| Recommended analytes |
|
|
|
|
| Intended purpose |
|
|
|
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 Table includes a selection from XanTec’s full PAG sensor chip portfolio.
3 Maximum immobilization capacities are based on capture from a 100 µg/mL solution of Protein A-purified, pooled rabbit IgG in PBS, with 1 µRIU corresponding approximately to 1 RU.
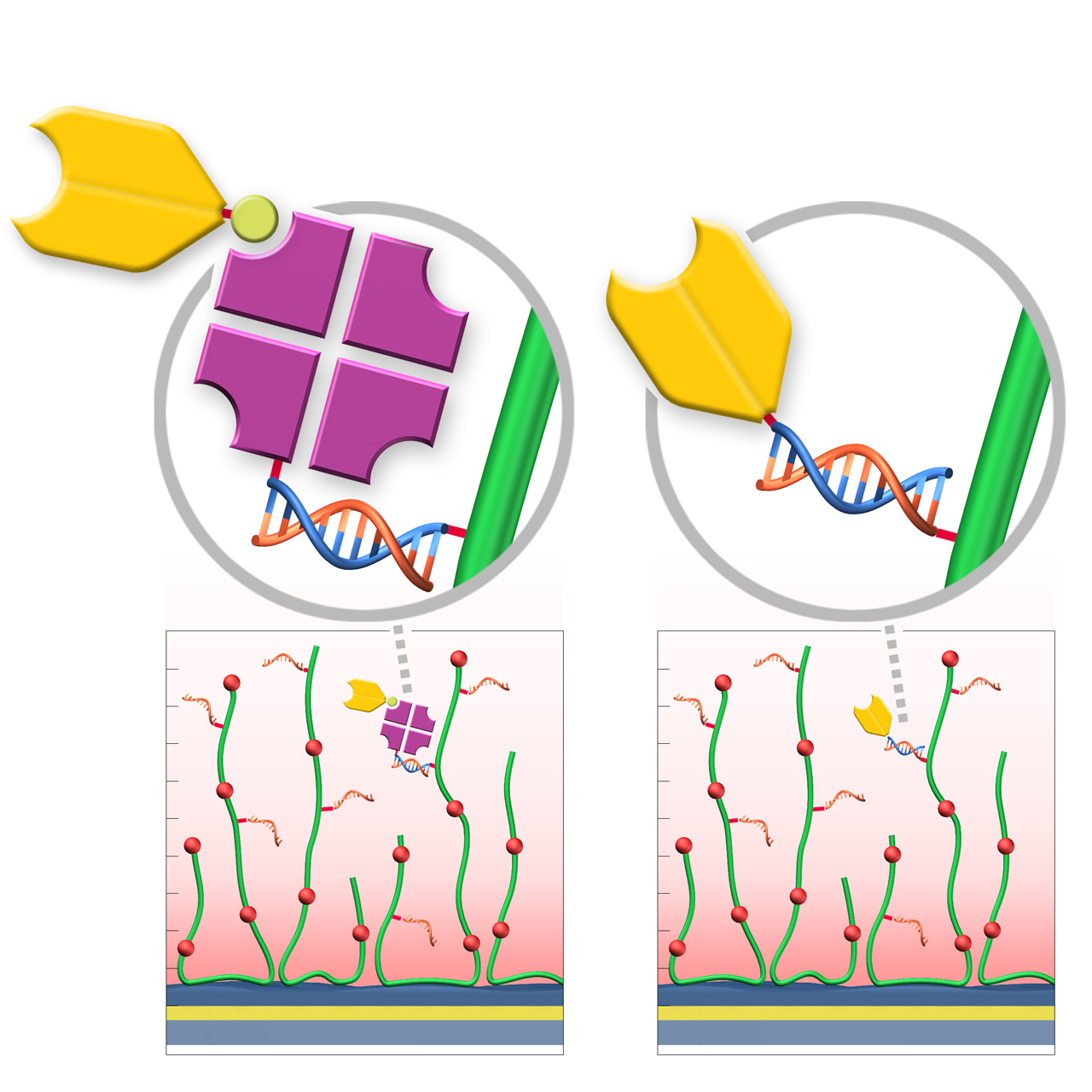
RG sensor chips (regenerable DNA-mediated capture)
XanTec’s RGD200M sensor chips are coated with a bioinert carboxymethyl-dextran matrix derivatized with a 24-nt single-stranded DNA oligonucleotide. This surface enables DNA-mediated, reversible ligand immobilization via hybridization with a complementary DNA sequence. Captured ligands are stably bound under physiological conditions, while quantitative regeneration is achieved by alkaline denaturation of the double-stranded DNA, restoring the surface for subsequent capture cycles.
Two complementary immobilization strategies are available. In Variant 1 (RG-SA), streptavidin pre-conjugated with the complementary DNA oligo is first immobilized via DNA hybridization, followed by capture of biotinylated ligands. In Variant 2 (RG-Modifier), ligands are directly conjugated to the complementary DNA oligo (e.g., via DBCO Click chemistry) and immobilized without an intermediate streptavidin step. Variant 2 can reduce nonspecific binding, lower diffusion limitations, enable higher capture densities, and facilitate faster assay setup.
Key features:
- Very fast assay development: No preconcentration, no surface activation, and no regeneration screening required; immobilization under physiological conditions.
- Maximum flexibility: Regenerable alternative to classic streptavidin/biotin capture immobilization.
- Exceptional stability: Drift-free immobilization of oligo-modified streptavidin.
- Simple regeneration: Quantitative regeneration enabling >100 capture/regeneration cycles.
- Oriented immobilization: Controlled biotinylation (e.g., AviTag™) supports high ligand activity and reproducibility.
- Ideal for large screening campaigns: Consistent surface performance across many cycles and sensor chips.
| Product code | RGD200M | RGD200M |
|---|---|---|
| Base coating | 3D, 200 nm bioinert CM-dextran (medium density) | |
| Variant | Variant 1: RG-SA | Variant 2: RG-Modifier |
| Capture immobilization capacity [µRIU] | ≈ 3,5002 | ≈ 5,0003 |
| Recommended ligands | biotinylated proteins or peptides | proteins or peptides with NHS-reactive amino groups |
| Recommended analytes |
|
|
| Intended purpose |
|
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 Based on capture of 100 µg/mL biotinylated BSA in PBS (RG-SA pre-capture), with 1 µRIU corresponding approximately to 1 RU.
3 Based on capture of 200 nM RG-SA on an RGD200M sensor chip.
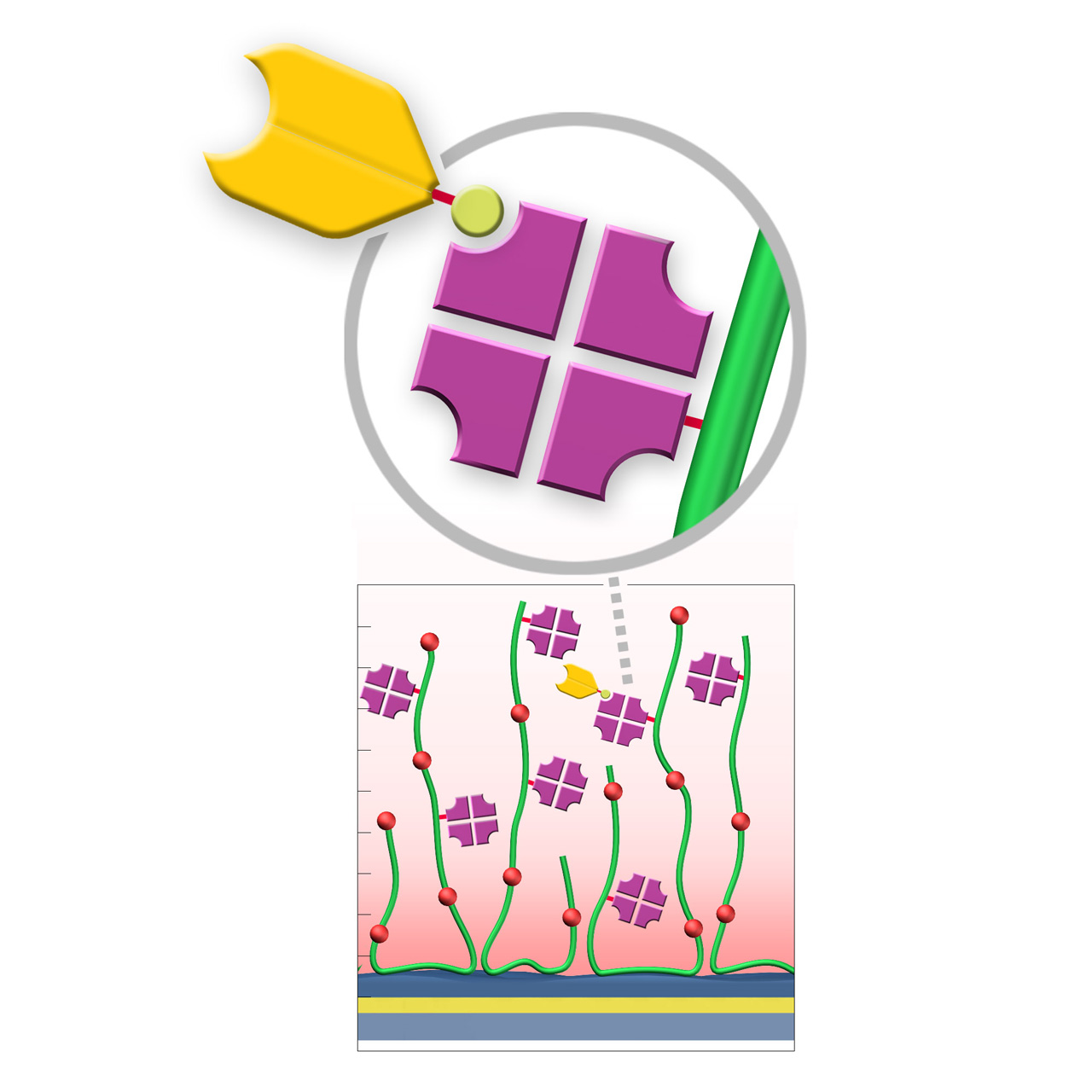
Streptavidin–modified sensor chips
XanTec’s streptavidin-modified (SA) sensor chips are coated with a bioinert (poly)carboxylate matrix pre-functionalized with a recombinant 52 kDa streptavidin tetramer. The immobilized streptavidin captures biotinylated biomolecules—including proteins, peptides, nucleic acids, and other biotin-tagged ligands—efficiently under physiological conditions. Owing to the exceptionally high affinity of streptavidin for biotin (dissociation constant ≈ 10−15 M), SA sensor chips enable highly stable ligand immobilization with negligible dissociation.
A general limitation of streptavidin-based capture surfaces is that the maximum achievable ligand density is typically lower than that obtained by direct covalent immobilization strategies combined with electrostatic preconcentration. This is because the pre-immobilized streptavidin tetramer occupies a significant fraction of the accessible volume within the evanescent field. This should be considered when high ligand densities are required, for example in interaction analyses involving small analytes.
Key features:
- Fast assay development: No preconcentration or surface activation required; controlled ligand immobilization under physiological conditions.
- Exceptional stability: The strong streptavidin–biotin interaction renders the surface resistant to most common regeneration protocols.
- Oriented immobilization: Controlled biotinylation strategies (e.g., AviTag™) ensure high ligand activity and reproducibility.
- Broad application range: Suitable for protein interaction studies, nucleic acid hybridization, antibody screening, and other biosensing applications.
- Versatile capture capacity: Available on various CMD and HC base coatings to match specific experimental needs.
| Product code | SAP | SAD200M | SAD700L | SAHC30M | SAHC200M | SAHC1500M |
|---|---|---|---|---|---|---|
| Base coating | 2D, ultra-short CM-dextran (high density) |
3D, 200 nm bioinert CM-dextran (medium density) |
3D, 700 nm bioinert CM-dextran (low density) |
3D, 30 nm bioinert polycarboxylate (medium density) |
3D, 200 nm bioinert polycarboxylate (medium density) |
3D, 1500 nm bioinert polycarboxylate (medium density) |
| Capture immobilization capacity [µRIU]3 | ≈ 600–1,200 | ≈ 4,000–5,000 | ≈ 6,000–8,000 | ≈ 1,200–1,800 | ≈ 3,500–5,000 | ≈ 4,500–6,000 |
| Recommended ligands | biotinylated
|
biotinylated
|
||||
| Recommended analytes |
|
|
|
|
|
|
| Intended purpose |
|
|
|
|
|
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 Table includes a selection from XanTec’s full SA sensor chip portfolio.
3 Based on capture of 100 µg/mL biotinylated BSA in PBS, with 1 µRIU corresponding approximately to 1 RU.
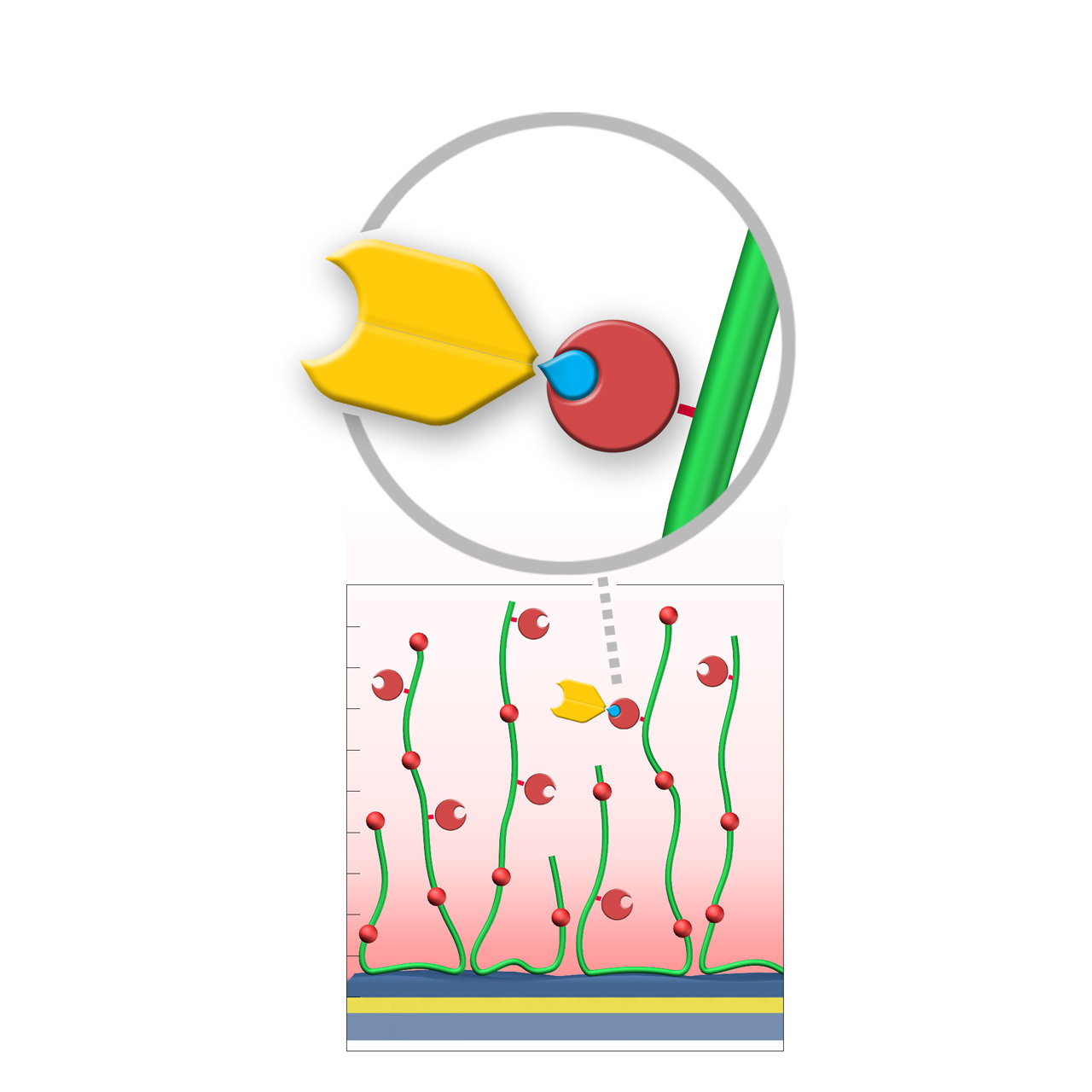
SpyCatcher–modified sensor chips
XanTec’s SpyCatcher (SPY) sensor chips are coated with a bioinert (poly)carboxylate matrix pre-functionalized with recombinant 12.8 kDa SpyCatcher3™. Immobilized SpyCatcher3 forms a highly stable covalent isopeptide bond with Spy-tagged proteins under physiological conditions (pH 5–8, 4–37 °C), enabling site-directed and permanent ligand immobilization.
Compared with the widely used streptavidin/biotin system, the SpyCatcher/SpyTag technology offers two major advantages. First, the interaction is truly covalent, resulting in exceptional long-term stability and drift-free baselines. Second, SpyCatcher3 is considerably smaller than streptavidin, occupying less volume within the hydrogel matrix and allowing higher ligand capture densities. These properties make SPY sensor chips particularly attractive for applications involving short peptides, small molecules, and fragments.
SPYD200M and SPYHC200M sensor chips provide high immobilization capacities for dense ligand presentation, while SPYP sensor chips combine lower capacity with excellent diffusion characteristics typical of 2D coatings. This makes SPYP surfaces especially suitable for protein–protein interaction studies and systems with fast association and dissociation rates.
Key features:
- Fast assay development: No preconcentration or chemical activation required; ligand immobilization occurs directly under physiological conditions.
- Exceptional stability: Covalent SpyCatcher/SpyTag coupling ensures permanently immobilized, drift-free ligands.
- Oriented immobilization: Site-directed attachment via N- or C-terminal SpyTag maximizes ligand activity.
- Versatile capture capacity: Available on CMDP, CMD, and HC base coatings to match specific experimental requirements.
| Product code | SPYP | SPYD200M | SPYHC200M |
|---|---|---|---|
| Base coating | 2D, ultra-short CM-dextran (high density) |
3D, 200 nm bioinert CM-dextran (medium density) |
3D, 200 nm bioinert polycarboxylate (medium density) |
| Capture immobilization capacity [µRIU]3 | ≈ 100 | ≈ 500 | ≈ 500 |
| Recommended ligands | Spy-tagged peptides and proteins | ||
| Recommended analytes |
|
|
|
| Intended purpose |
|
|
|
1 SpyCatcher3 carries a His-tag that may interact with certain ligands or analytes.
2 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
3 Based on capture of 100 nM SpyTag, with 1 µRIU corresponding approximately to 1 RU.
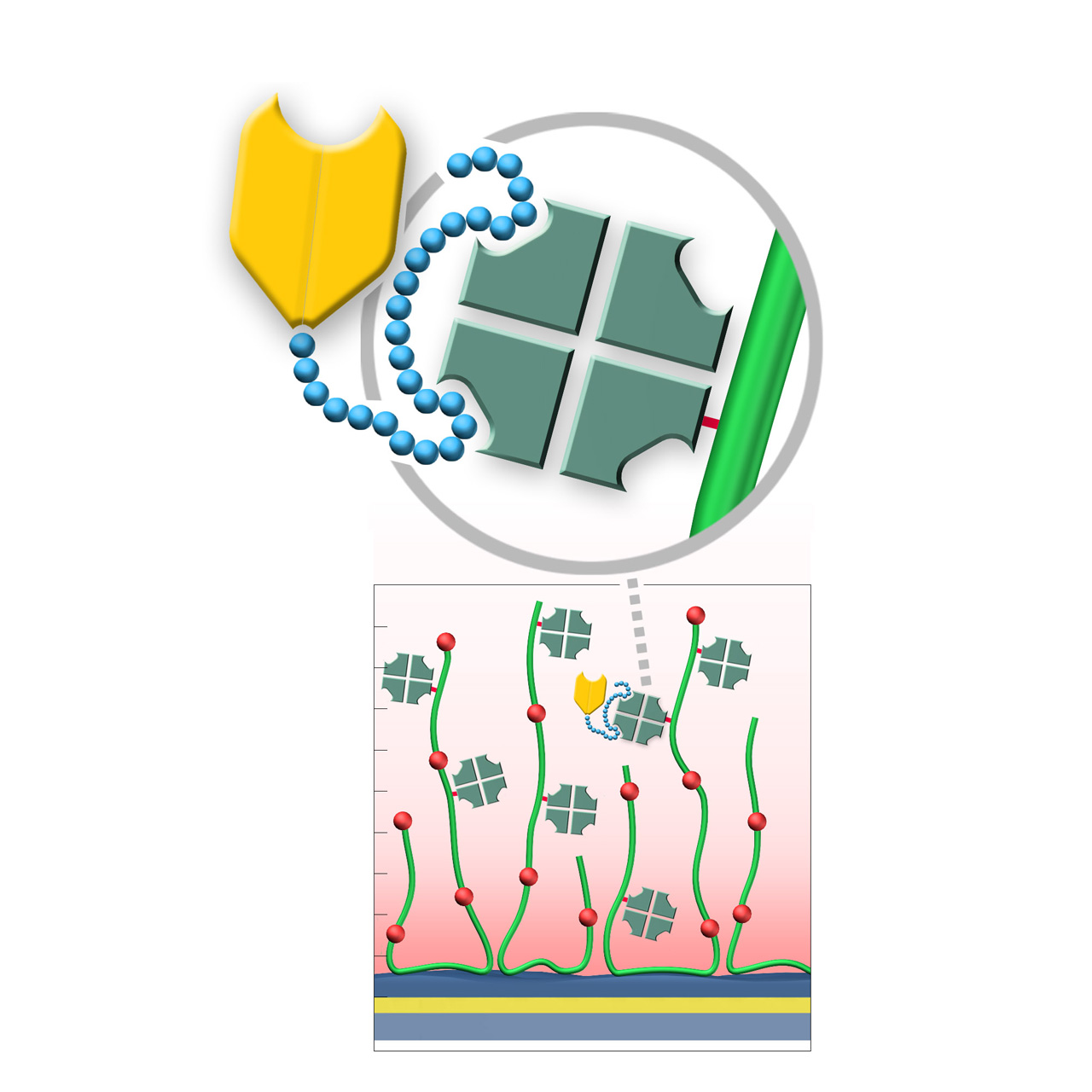
Strep-Tactin XT–modified sensor chips (ST)
XanTec’s Strep-Tactin XT–modified (ST) sensor chips are coated with a bioinert (poly)carboxylate matrix pre-functionalized with a recombinant 52 kDa Strep-Tactin XT tetramer. Although a member of the avidin family, Strep-Tactin XT exhibits only weak micromolar affinity for biotin and is therefore not suitable for stable immobilization of biotinylated biomolecules. Instead, it binds the 3 kDa Twin-Strep-Tag (TST) sequence with exceptionally high picomolar affinity, displaying typical dissociation rates (koff) of ≤ 10−5 s−11,2.
This enables defined, site-directed immobilization of TST fusion proteins under physiological conditions, ensuring uniform ligand orientation and preservation of biological activity. The Strep-Tactin XT/TST complex can be quantitatively regenerated using brief pulses of 3 M guanidine·HCl, with ST chips tolerating more than 100 regeneration cycles.
Compared with NTA/His-tag systems, ST chips exhibit markedly lower nonspecific binding of proteins and peptides, making them particularly suitable for kinetic and affinity studies involving these analytes.
XanTec offers three versions of Strep-Tactin XT–modified chips. The 2D STP chip provides superior diffusion properties, ideal for bulky analytes or weak binders with rapid kinetics. The STD200L and STHC200M chips enable higher immobilization densities for applications involving smaller analytes.
Key features:
- Fast assay development: No preconcentration or chemical activation required; ligand capture occurs under physiological conditions.
- Exceptional stability: Picomolar TST binding ensures virtually drift-free immobilization.
- Simple regeneration: Quantitative regeneration allows > 100 capture/regeneration cycles.
- Oriented immobilization: Uniform ligand orientation maximizes biological activity.
- Versatile capture capacity: Available on CMDP, CMD200L, and HC200M base coatings.
| Product code | STP | STD200L | STHC200M |
|---|---|---|---|
| Base coating | 2D, ultra-short CM-dextran (high density) | 3D, 200 nm bioinert CM-dextran (low density) | 3D, 200 nm bioinert polycarboxylate (medium density) |
| Capture immobilization capacity [µRIU]4 | ≈ 500–1,000 | ≈ 5,000–7,000 | ≈ 5,000–7,000 |
| Recommended ligands | TST-modified proteins and peptides | ||
| Recommended analytes |
|
|
|
| Intended purpose |
|
|
|
1 Apparent dissociation rate constants (koff) determined at ligand capture densities < 50% of maximum immobilization level.
2 Binding of Strep-Tag II is feasible but exhibits limited stability (koff ≈ 10−2–10−3 s−1).
3 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
4 Based on specific capture immobilization of 500 nM TST-GFP in PBS.
ZC–modified sensor chips (Zwitterionic)
XanTec’s ZC sensor chips are coated with a zwitterionic polymer comprising an approximately equimolar ratio of carboxylic acid and tertiary amine groups. The resulting near-neutral net charge renders the surface highly bioinert toward charge-driven nonspecific binding.
ZC sensor chips support efficient EDC/NHS-based covalent coupling through a transient charge-shift mechanism. During activation, negatively charged carboxyl groups are converted into charge-neutral NHS esters, shifting the net surface charge toward positive. In this activated state, reversed-charge electrostatic preconcentration occurs, favoring enrichment of negatively charged ligands. After coupling, hydrolysis or quenching (e.g., with glycine) restores the charge-neutral zwitterionic surface.
These properties make ZC sensor chips particularly suitable for immobilizing acidic ligands, ligands unstable under acidic preconcentration conditions, and for analyzing strongly cationic analytes that would otherwise bind nonspecifically to classic polycarboxylate coatings such as CMD or HC.
Key features:
- Acidic ligand coupling: Reversed-charge electrostatic preconcentration enables covalent amine coupling of acidic ligands (e.g., DNA, RNA) at near-neutral pH.
- Gentle immobilization conditions: Efficient coupling typically around pH 6.5, preserving activity of pH-sensitive biomolecules.
- Wide immobilization range: Several thousand (ZC30M) to ~30,000 µRIU (ZC150D).
- Exceptional bioinertness: Charge-neutral zwitterionic matrix minimizes nonspecific binding, even for highly cationic biomolecules.
- No polysaccharide backbone: Absence of carbohydrate motifs prevents unintended lectin interactions.
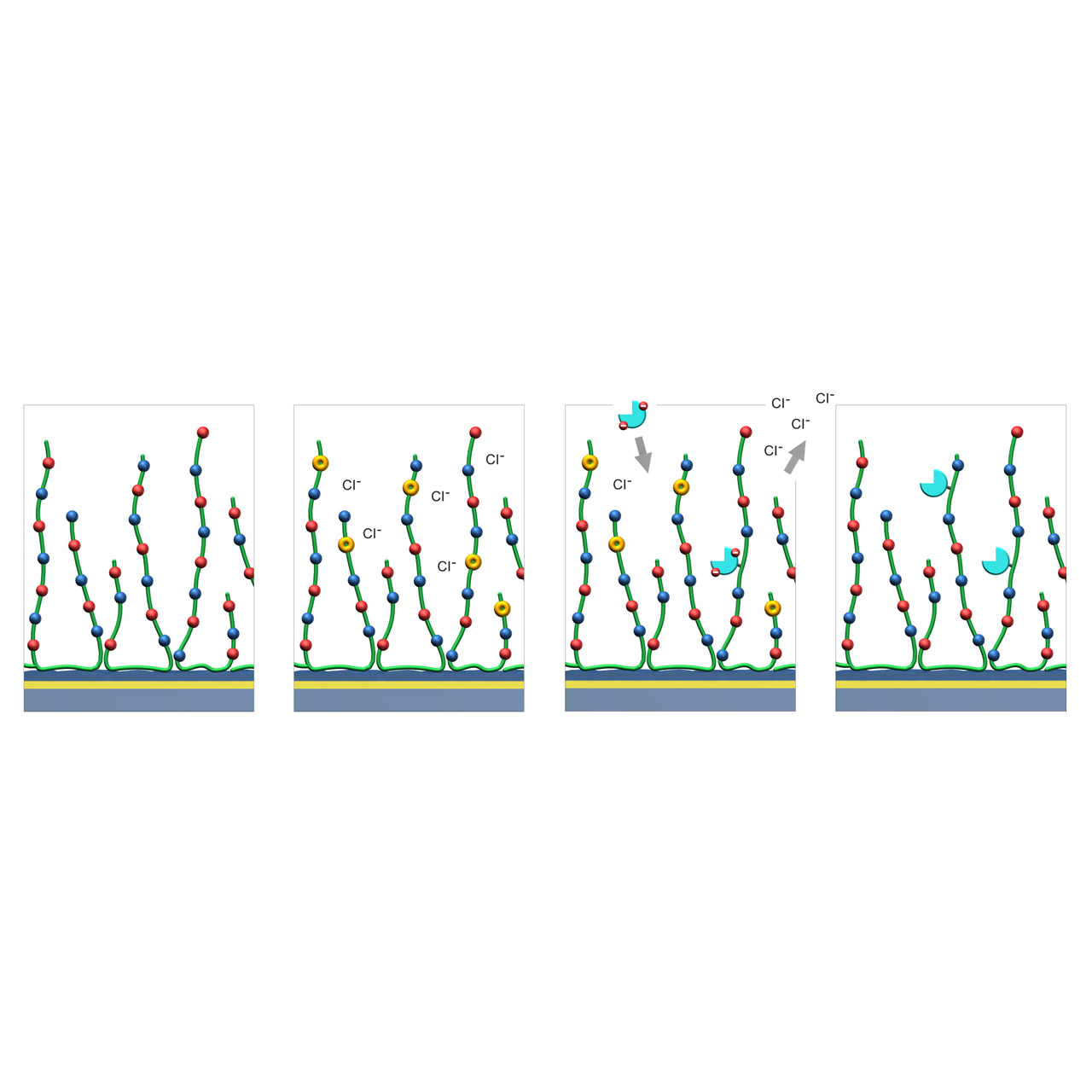
1. Native ZC sensor chip: Red and blue dots represent carboxyl and tertiary amine groups evenly distributed along the green polymer chains.
2. EDC/NHS activation: A fraction of the negatively charged carboxyl groups are converted into charge-neutral NHS esters (yellow), transiently shifting the net surface charge toward positive.
3. Reversed-charge preconcentration and coupling: Ligands with a negative net charge (cyan) accumulate at the surface via reversed-charge electrostatic preconcentration and are subsequently covalently immobilized through formation of amide bonds.
4. Deactivation: Hydrolysis or quenching (e.g., with 1 M glycine at pH 8.5) converts remaining NHS esters back into carboxylic acids, restoring the charge-neutral surface.
| Product code | ZC30M | ZC80M | ZC150D | ZCC150D |
|---|---|---|---|---|
| Base coating | 3D, 30 nm bioinert charge-neutral zwitterionic polymer (medium density) | 3D, 80 nm bioinert charge-neutral zwitterionic polymer (medium density) | 3D, 150 nm bioinert charge-neutral zwitterionic polymer (high density) | 3D, 150 nm bioinert charge-positive zwitterionic polymer (high density) |
| Covalent immobilization capacity [µRIU]2 | ≈ 2,600 (3,000) | ≈ 6,000 (7,000) | ≈ 22,000 (30,000) | ≈ 0 (30,000) |
| Recommended ligands3 |
|
|||
| Recommended analytes |
|
|
|
|
| Intended purpose |
|
|
|
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 Typical covalent immobilization capacity and electrostatic preconcentration capacity (in brackets) determined by injecting 100 µg/mL Protein A in 10 mM MES buffer (pH 6.5) following surface activation with 0.5 M EDC in NHS/MES activation buffer.
3 All ligands require at least one NHS-reactive amino group.
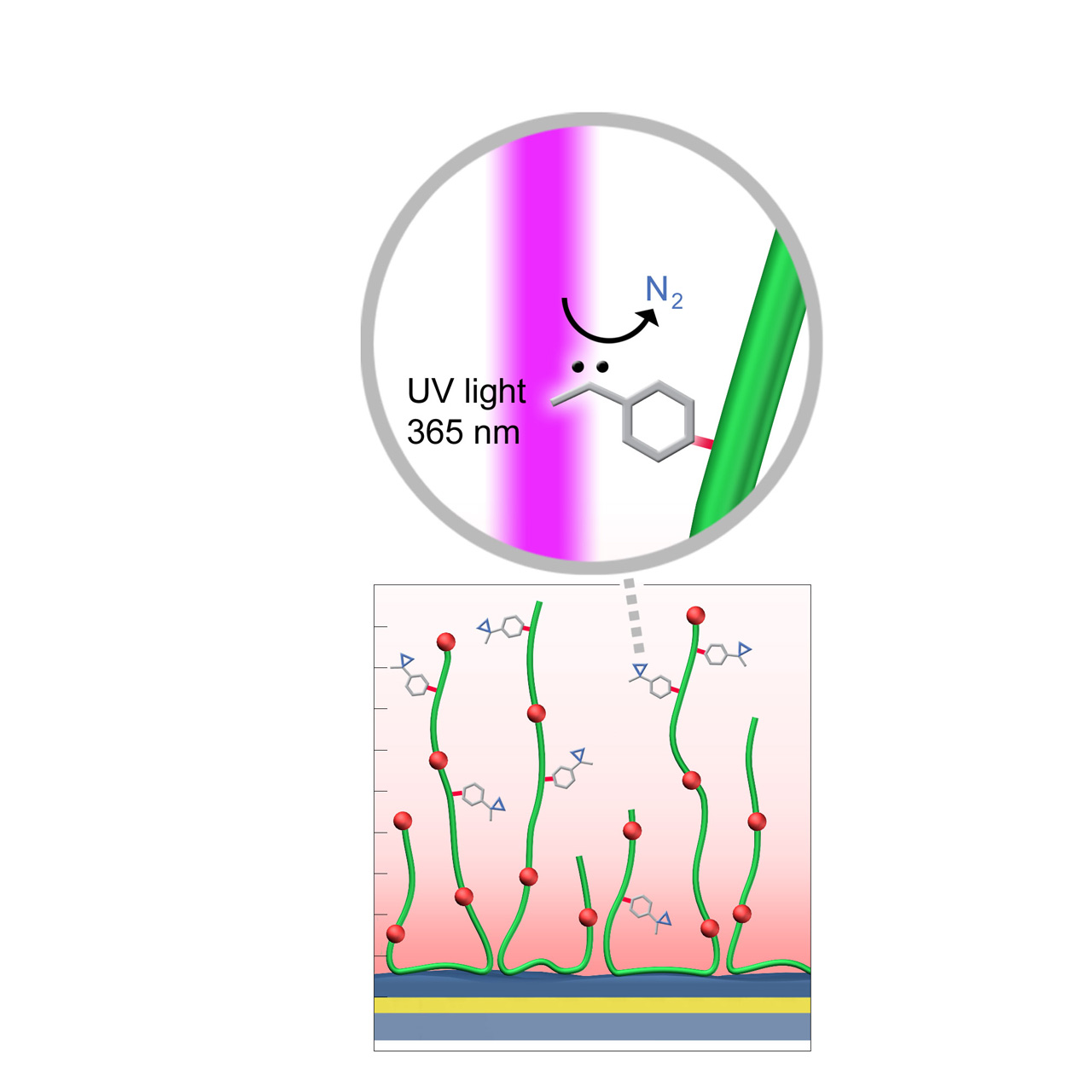
UV (Diazirine)–modified sensor chips
XanTec’s UV sensor chips are available as a 2D coating (UVHCP) or as 3D hydrogel matrices (UVHC200M, UVD200M) pre-functionalized with low-molecular-weight diazirine groups grafted onto a hydrophilic adhesion promoter on a gold support. Diazirines are stable in the dark but, upon irradiation at 365 nm, undergo photolysis, releasing N2 and generating a highly reactive carbene. This carbene inserts into thiol, amino, hydroxy, and even C–H bonds, enabling stable covalent attachment to virtually any biomolecule.
When combined with electrostatic preconcentration, UV coupling provides a robust and efficient immobilization strategy. Efficient immobilization requires direct UV light access to the sensor surface, which may necessitate a quartz flow cell window. In combination with microarray spotters, UV-activated coupling can also be used to generate highly defined microarray sensor chips or slides.
Key features:
- Robust covalent ligand coupling: Low chemical selectivity allows covalent attachment of nearly any biomolecule without additional reagents.
- Compatible with spot immobilization: Particularly suitable for microarray spotting and imaging-SPR applications; high coupling yields achievable after drying prior to UV activation.
- Requires special equipment: UV-permeable (quartz) flow cells are required for in-flow activation.
| Product code | UVHCP | UVHC200M | UVD200M |
|---|---|---|---|
| Base coating | 2D, ultra-short bioinert polycarboxylate (high density) |
3D, 1000 nm bioinert polycarboxylate (medium density) |
3D, 200 nm bioinert CM-dextran (medium density) |
| Covalent immobilization capacity [µRIU]2 | ≈ 1,500 | ≈ 11,000 | ≈ 10,000 |
| Recommended ligands |
|
|
|
| Recommended analytes |
|
|
|
| Intended purpose |
|
|
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 Covalent immobilization capacity determined by injecting 100 µg/mL BSA in 5 mM sodium acetate buffer (pH 5.0) and irradiating for 10 min with a 3 W, 365 nm UV LED using a quartz window flow cell.
CMPEG–modified sensor chips
XanTec’s CMPEG sensor chips (not to be confused with CMPG) are based on short, 4 kDa carboxymethyl-terminated polyethylene glycol (PEG) chains grafted onto a hydrophilic adhesion promoter on a gold support. Ligands can be covalently attached via their amine groups using EDC/sulfo-NHS-based coupling chemistry1, enabling immobilization of proteins, antibodies, peptides, nucleic acids, carbohydrates, and small molecules.
Because PEG chains terminate in a single reactive carboxyl group, protein immobilization capacity is limited to a near-monolayer and is therefore relatively low (typically ~1,000–2,000 µRIU). At the same time, this minimal and well-defined surface architecture provides a high degree of conformational freedom for immobilized ligands, helping to preserve biological activity. The amphiphilic PEG backbone reduces nonspecific binding.
CMPEG sensor chips are frequently requested because PEG is considered a bioinert coating, particularly when dextran-based derivatives such as CMD cannot be applied. However, alternative XanTec coatings (e.g., HCP, CMTEG, CMPG) may offer superior performance in applications requiring maximum surface inertness, as CMPEG surfaces can exhibit elevated nonspecific adsorption under certain conditions.
Key features:
- Well-known sensor matrix: Short CMPEG brush with well-characterized surface chemistry and moderate nonspecific binding, including for highly cationic biomolecules.
- Versatile ligand coupling: Supports covalent attachment via established EDC/sulfo-NHS chemistry.
- Low sensor-matrix profile: Terminally exposed carboxyl groups maximize ligand accessibility and support high ligand activity despite low immobilization capacity.
- No polysaccharide backbone: Absence of carbohydrate motifs prevents unintended interactions with lectins and other carbohydrate-binding biomolecules.
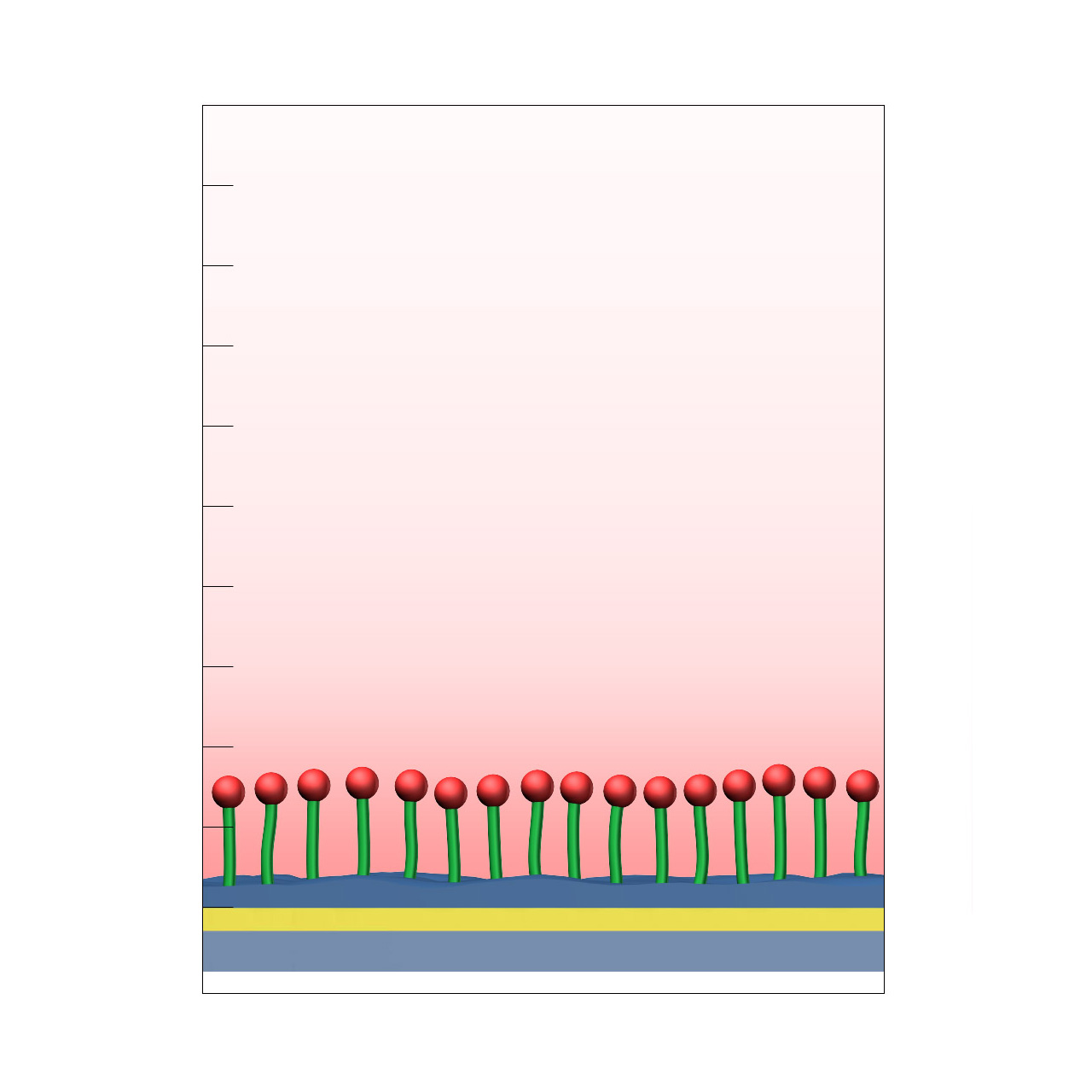
| Product code | CMPEG |
|---|---|
| Base coating | Dense 4 kDa PEG polymer brush with terminal carboxyl groups |
| Electrostatic preconcentration capacity [µRIU]3 | ≈ 1,000–2,000 |
| Recommended ligands |
|
| Recommended analytes |
|
| Intended purpose |
|
1 Use of sulfo-NHS is recommended for efficient protein coupling due to the low surface charge density of CMPEG coatings.
2 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
3 Preconcentration capacity determined by injecting 100 µg/mL BSA in 5 mM sodium acetate pH 5.0, with 1 µRIU corresponding to ≈ 1 RU. Maximum covalent coupling efficiencies typically span ≈ 20–45% of the electrostatic preconcentration capacity and depend strongly on protein properties.
Polylysine–modified sensor chips (PLY)
XanTec’s Polylysine (PLY) sensor chip is based on a 3D poly-L-lysine hydrogel matrix grafted onto a hydrophilic adhesion promoter on a gold support. In contrast to anionic carboxymethyl-dextran (CMD) or linear polycarboxylate (HC) coatings, the PLY matrix is strongly polycationic and therefore exhibits distinct physicochemical properties.
PLY surfaces are permanently positively charged and support electrostatic preconcentration of anionic ligands. Despite their polycationic nature, nonspecific interactions in physiological buffers are typically moderate. Because the surface lacks carboxyl groups, standard EDC/NHS activation followed by amine coupling is not applicable. Covalent immobilization must rely on alternative chemistries, such as coupling of NHS-preactivated anionic ligands (e.g., COOH-modified oligonucleotides). Even under physiological conditions, electrostatic interactions with polyanionic species may occur. For these reasons, PLY sensor chips are not typically the first choice for routine SPR-based kinetic or affinity measurements.
Nevertheless, the PLY matrix provides a useful bioinert polycationic reference coating for evaluating nonspecific interactions and benchmarking newly developed biomedical or antifouling surface chemistries.
Key features:
- Immobilization of anionic ligands: The polycationic matrix supports preconcentration and immobilization of NHS-activated anionic ligands such as DNA oligonucleotides.
- Reference surface: Provides a relatively bioinert polycationic hydrogel suitable for assessing nonspecific binding and benchmarking novel biomedical coating materials.
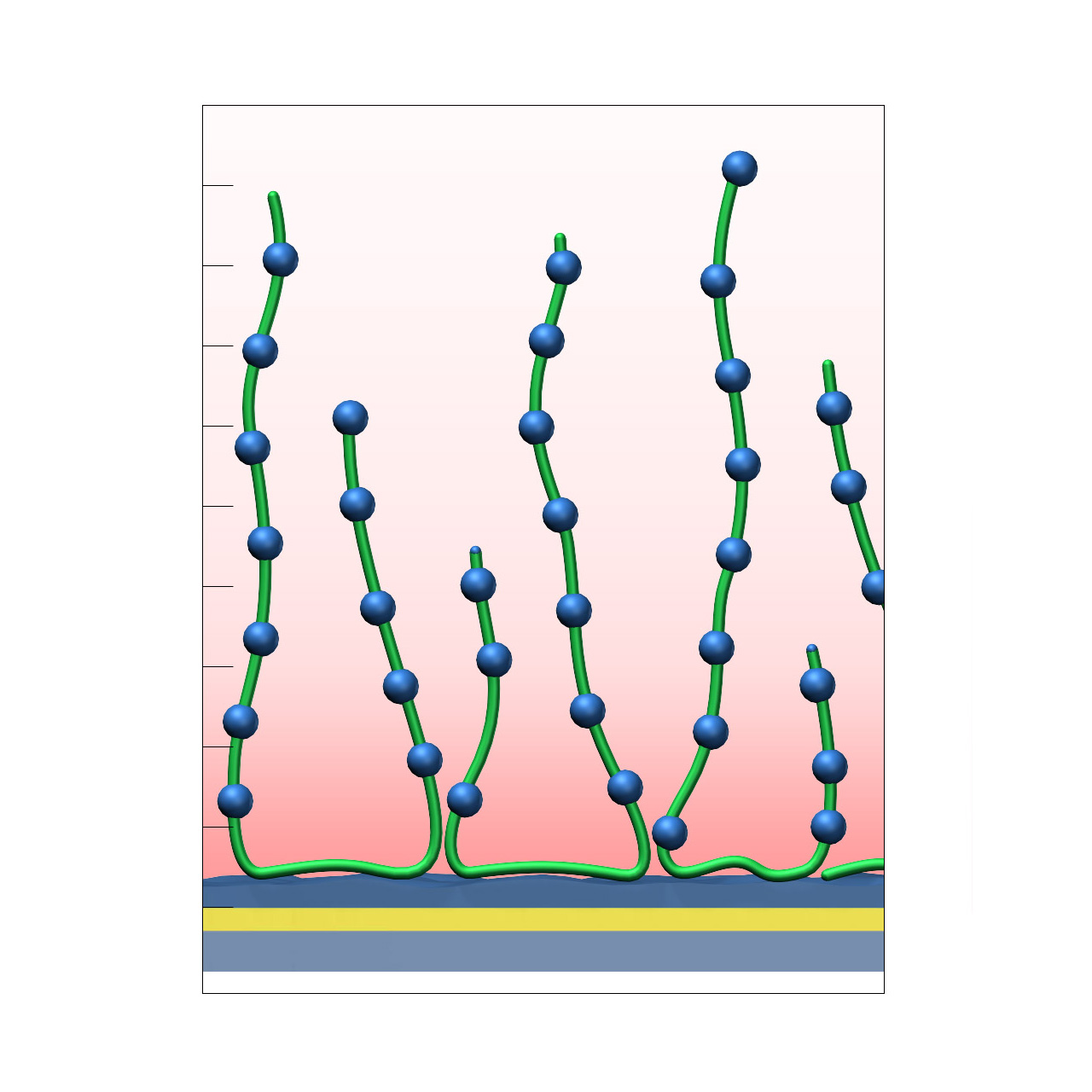
| Product code | PLY |
|---|---|
| Base coating | 3D, medium-length bioinert polylysine (medium density) |
| Electrostatic preconcentration capacity [µRIU]2 | ≈ 40,000 |
| Recommended ligands | NHS-preactivated acidic ligands |
| Recommended analytes | — |
| Intended purpose |
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 Preconcentration capacity determined by injecting 100 µg/mL bovine serum albumin (BSA) in 5 mM sodium acetate pH 5.0, with 1 µRIU corresponding to ≈ 1 RU.
CMTEG–modified sensor chips
XanTec’s 2D CMTEG sensor chips are based on an ultra-short, carboxymethylated tetraethyleneglycol (CMTEG) brush grafted onto a hydrophilic adhesion promoter on a gold support. Ligands can be covalently attached via their amine, thiol, or aldehyde groups using established coupling chemistries such as EDC/sulfo-NHS activation, thiol–maleimide coupling, or reductive amination. This enables immobilization of proteins, antibodies, peptides, nucleic acids, carbohydrates, and small molecules.
Protein immobilization capacity is relatively low (typically < 1000 µRIU), while the highly hydrophilic surface provides outstanding resistance to nonspecific binding. In contrast to the chemically related CMPEG surface, CMTEG is not amphiphilic due to its much shorter polyethylene chains. As a result, CMTEG surfaces are particularly useful in experiments challenged by persistent nonspecific interactions, especially those involving strongly positively charged biomolecules or complex sample matrices such as cell culture media.
Key features:
- Very low background binding: Excellent suppression of nonspecific binding, particularly highly cationic biomolecules; well suited for complex sample matrices such as cell culture media.
- Versatile ligand coupling: Supports covalent attachment via amine, thiol, or aldehyde groups using established chemistries (EDC/sulfo-NHS, maleimide, reductive amination).
- Low sensor matrix profile: The planar 2D coating enables reliable kinetic analysis of systems with fast on- and off-rates.
- No polysaccharide backbone: Absence of carbohydrate motifs prevents unwanted interactions with lectins and other carbohydrate-binding biomolecules.
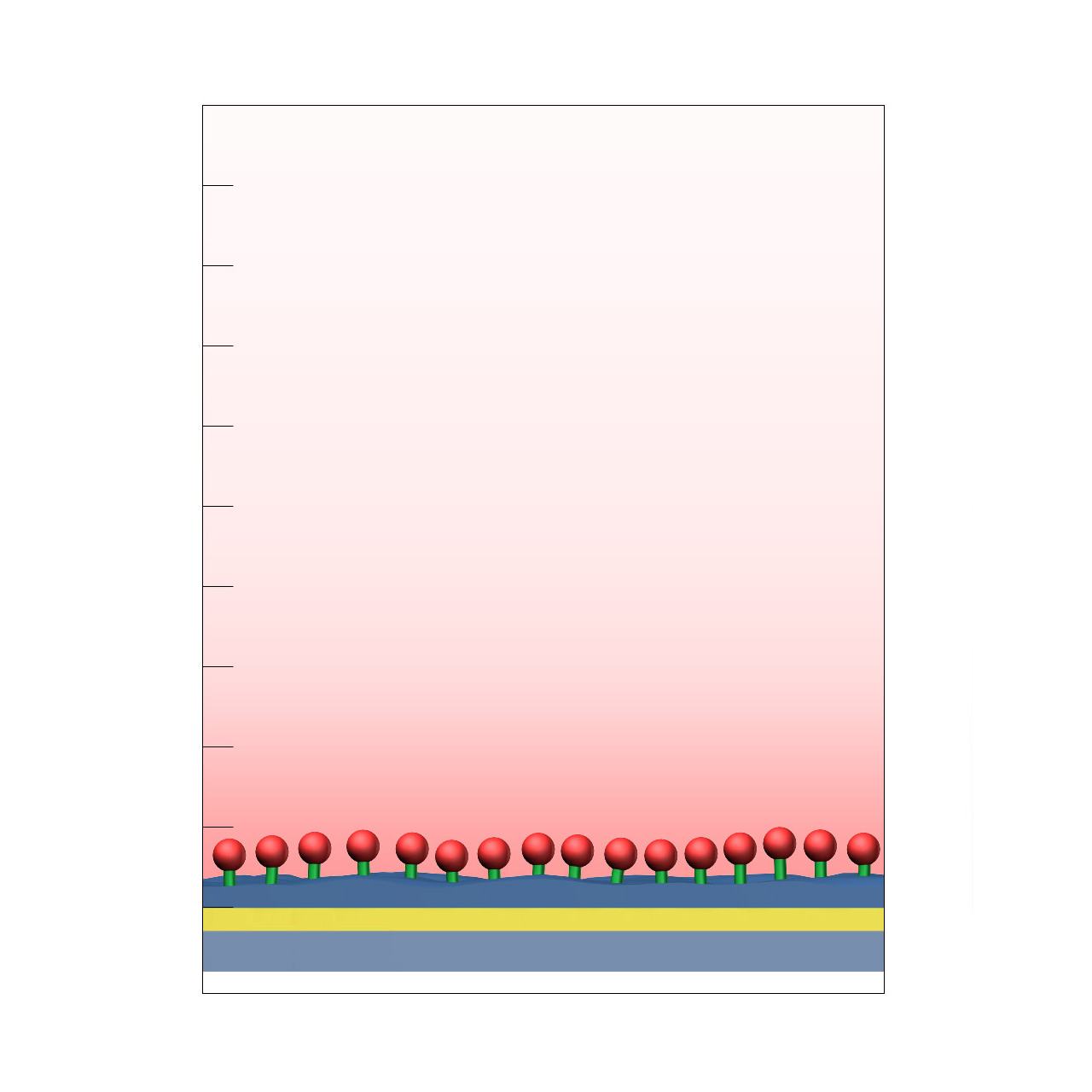
| Product code | CMTEG |
|---|---|
| Base coating | 2D, CM-tetraethyleneglycol (high density) |
| Electrostatic preconcentration capacity [µRIU]2 | < 1000 |
| Recommended ligands |
|
| Recommended analytes |
|
| Intended purpose |
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 Preconcentration capacity determined by injecting 100 µg/mL bovine serum albumin (BSA) in 5 mM sodium acetate pH 5.0, with 1 µRIU corresponding to ≈ 1 RU. Maximum covalent coupling yields depend strongly on the properties of the immobilized protein; under optimal conditions, typical coupling efficiencies span ≈ 20–45% of the respective electrostatic preconcentration capacity.
AU sensor chips
XanTec’s AU sensor chips consist of an approximately 44 nm gold layer deposited on a borosilicate glass substrate. These chips are intended for custom surface development or use as a reference surface.
Direct adsorption-based ligand immobilization is generally not recommended, as it frequently results in partial ligand denaturation and elevated nonspecific background signals, limiting suitability for standard SPR interaction analyses.
Key features:
- Base sensor chip: Provides a clean, well-defined gold surface suitable for development and evaluation of custom surface chemistries.
- Surface conductive for Electro-SPR (E-SPR): Enables electrochemical SPR applications.
- Reference chip: Serves as a control surface for assessing nonspecific interactions or benchmarking newly developed coatings.
| Product code | Au |
|---|---|
| Base coating | Bare gold, ≈ 44 nm, on borosilicate glass support |
| Intended purpose |
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
AU HRI sensor chips
XanTec’s AU HRI sensor chips consist of an approximately 44 nm gold layer deposited on a high-refractive-index (HRI) glass substrate (SF10, n = 1.72827 @ 588 nm). These chips are intended for custom surface development or use as a reference surface.
Direct adsorption-based ligand immobilization is generally not recommended, as it frequently results in partial ligand denaturation and elevated nonspecific background signals, limiting suitability for standard SPR interaction analyses.
Key features:
- Base sensor chip: Provides a clean, well-defined gold surface suitable for development and evaluation of custom surface chemistries.
- Surface conductive for Electro-SPR (E-SPR): Enables electrochemical SPR applications.
- Reference chip: Serves as a control surface for assessing nonspecific interactions or benchmarking newly developed coatings.

| Product code | AU HRI |
|---|---|
| Base coating | Bare gold, ≈ 44 nm, on SF10 glass support (n = 1.72827 @ 588 nm) |
| Intended purpose |
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
QZ sensor chips (SiO2-coated)
XanTec’s QZ sensor chips are coated with an approximately 10 nm silicon dioxide (SiO2) layer deposited on a 44 nm gold film. These chips are intended for lipid bilayer immobilization, custom surface development on oxidic substrates, or use as a reference surface.
Direct adsorption-based ligand immobilization is not recommended, as it typically results in low immobilization capacity, partial denaturation of protein ligands, and elevated nonspecific background signals, limiting suitability for standard SPR interaction analyses.
Please note that the deposited SiO2 layer induces a substantial SPR signal offset of approximately 20,000 µRIU, which should be considered during experimental setup and data interpretation.
Key features:
- Base sensor chip: Provides a dense, well-defined SiO2 surface suitable for development and evaluation of custom surface chemistries on oxidic materials.
- Reference chip: Serves as a control surface for assessing nonspecific interactions or benchmarking newly developed sensor coatings.
| Product code | QZ |
|---|---|
| Base coating | SiO2, ≈ 10 nm, on gold support |
| Intended purpose |
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
Dextran–modified sensor chips
XanTec’s Dextran (D) sensor chips are available as a 2D dextran coating (DP) or as a 3D hydrogel matrix (D200M) composed of unmodified dextran chains grafted onto a hydrophilic adhesion promoter on a gold support. In contrast to carboxymethyl-dextran (CMD) coatings, D-type surfaces carry no charge and therefore do not exhibit electrostatic interactions and do not support electrostatic preconcentration.
Because the surface lacks carboxyl groups, standard EDC/NHS amine coupling is not applicable. Covalent immobilization must rely on alternative chemistries, such as oxidation of dextran to polyaldehydes followed by Schiff base formation / reductive amination, or (bis)epoxy activation. As no electrostatic enrichment occurs, higher ligand concentrations are often required. For these reasons, Dextran sensor chips are not typically the first choice for routine SPR-based kinetic or affinity measurements.
Nevertheless, the absence of charged functional groups provides the surface with exceptionally low nonspecific binding, making D sensor chips a versatile basis for individual immobilization chemistries. Furthermore, D sensor chips can be used as bioinert, low drifting reference coatings for instrument hardware tests, to evaluate nonspecific interactions or to assessnew biomedical and antifouling surface chemistries.
Key features:
- Exceptionally bioinert: The charge-free dextran matrix exhibits extremely low nonspecific binding.
- Protocol compatibility: The polysaccharide matrix is similar to common bioseparation media (e.g., Sephadex), enabling transfer of immobilization protocols developed for crosslinked polysaccharide beads.
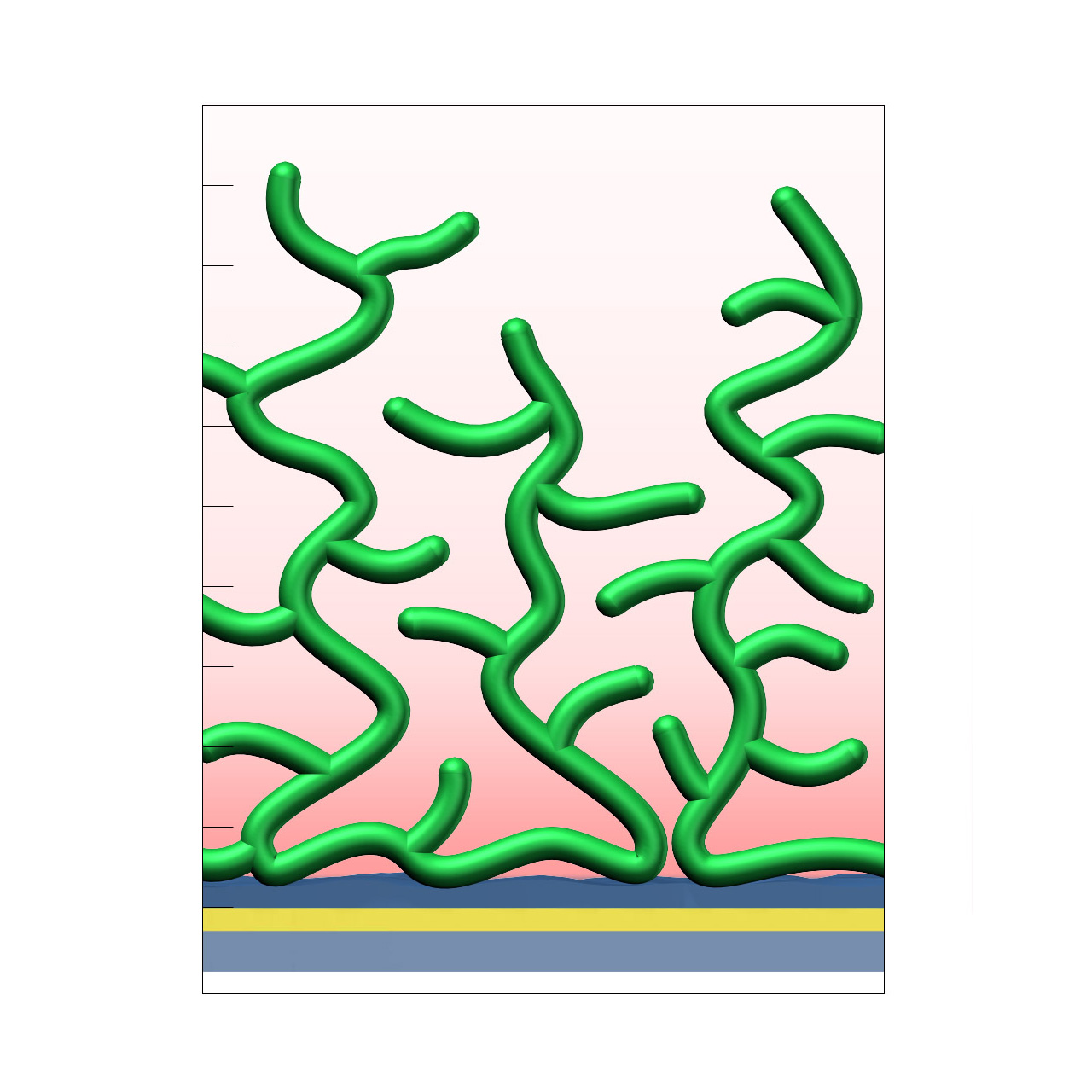
| Product code | DP | D200M |
|---|---|---|
| Base coating | 2D, ultra-short bioinert dextran (high density) |
3D, 200 nm bioinert dextran (medium density) |
| Immobilization capacity [µRIU] | Not available | Not available |
| Recommended ligands | Not available | Not available |
| Recommended analytes | Not available | Not available |
| Intended purpose |
|
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
Agarose
Agarose (MW approx. 120 kDa) monolayer grafted to a thin adhesion promoter.
Linear polymer consisting of alternating D-galactose and 3,6-anhydro-L-galactopyranose linked by α-(1→3) and β-(1→4) glycosidic bonds.
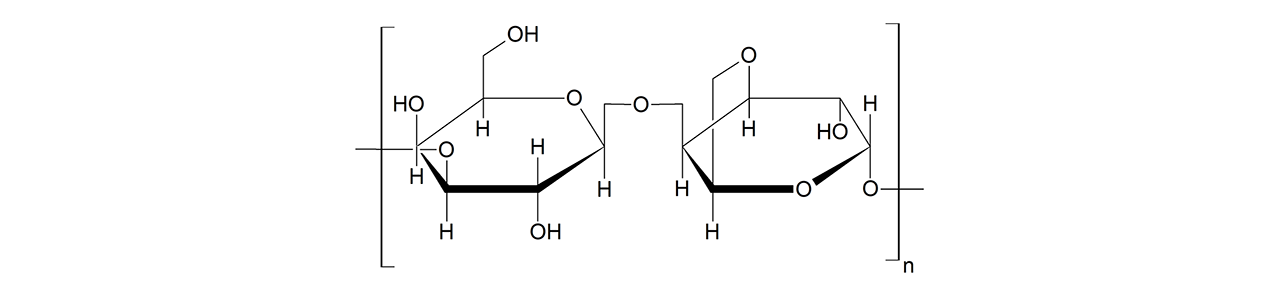
Click chemistry (AZC, AZD, DCD)
Click Chemistry or Click Coupling has gained increasing popularity among chemists because of its outstanding reaction kinetics, excellent selectivity, and superior stability in physiological environments. To make this bioconjugation technology available for ligand immobilization in SPR biosensing, polycarboxylate sensor coatings are modified with a very small, bioinert, azido (N3) group, while the ligand is labeled with a low-molecular-weight cyclooctyne linker (DBCO), a procedure comparable to ligand biotinylation. These groups can react in a quantitative and highly-selective manner via a simple alkyne–azide cycloaddition, forming a stable, covalent triazole linkage.
Some expression systems are capable of site-specific integration of non-natural amino acids containing an azide moiety. For efficient, selective and site-directed Click Coupling of such ligands XanTec also offers DBCO-modified sensor chips.
You can find more information about azide- and DBCO-modified polycarboxylate sensor chips for Click Chemistry here.
Linear polycarboxylate (C)
The robust C hydrogel consists of a hydrophilic, strictly linear polycarboxylate with a polyalkyl backbone. Compared with CM-Dextran (CMD) coatings, this hydrogel shows significantly lower diffusion limitation, resulting from its small molecular footprint and higher flexibility of the polymer chains. It has a high carboxyl group density and is a good choice if the ligand and analyte are small and high immobilization density is required. Due to the high negative charge density, the C coating is particularly well suited for acidic analytes, such as DNA. Harsh conditions are tolerated as the C–C backbone withstands practically all aqueous buffers and organic solvents except strong oxidizing agents.
Chondroitin sulfate (CDS)
CDS is an unbranched glycosaminoglycan composed of a chain of alternating sugars (N-acetyl-D-galactosamine and D-glucuronic acid) where each sugar can be sulfated in variable positions and quantities.
As a mucopolysaccharide and component of cartilage tissue, CDS shows good biocompatibility while being mechanically relatively rigid. The abundant carboxyl groups allow for activation using standard EDC/NHS chemistry.
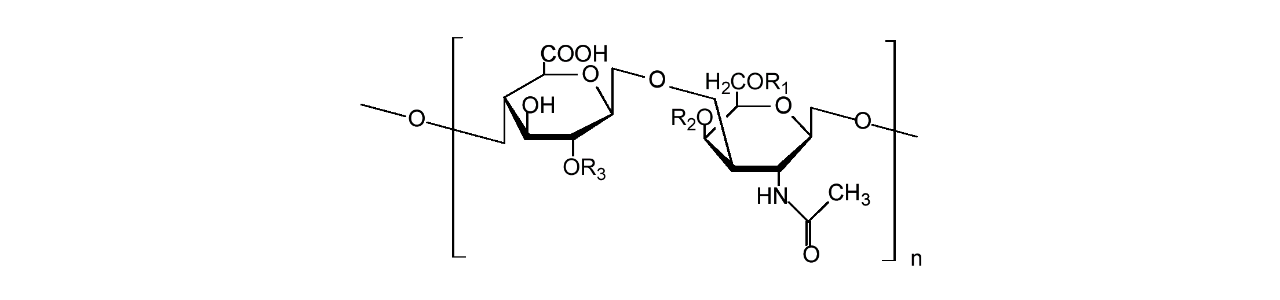
CM-Cellulose
Carboxymethyl cellulose (CM-Cellulose, CMC) is a cellulose derivative with carboxymethyl groups (–CH2–COOH) bound to hydroxyl groups of the glucopyranose monomers that make up the cellulose backbone. Dependent on the degree of carboxymethylation and the polymer length, functional properties of CMC vary. CMC sensor surfaces can be useful for interaction studies of plant derived biomolecules or cellulose-related research.
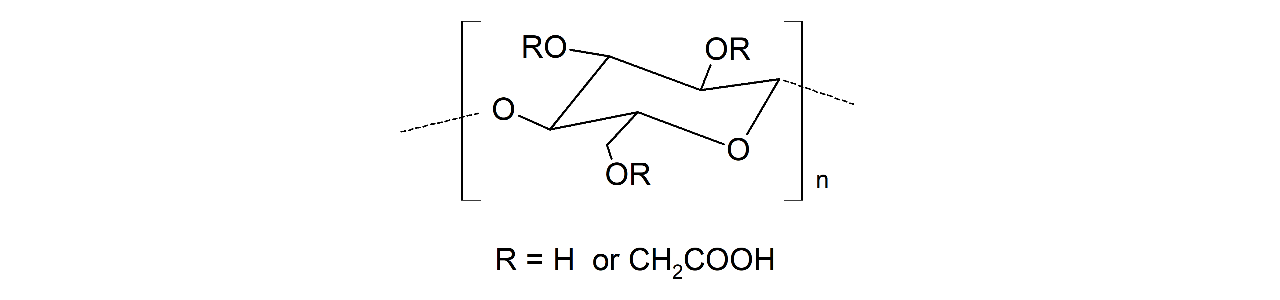
Sulfonated polycarboxylate hydrogel (CS)
The partially sulfonated “C“-matrix consists of a biocompatible, hydrophilic, strictly linear polycarboxylate comprising of a brush-structured polyalkyl polymer backbone in which some carboxylic groups have been replaced by more acidic sulfonates. Having a lower pKa compared with carboxylates, these sulfonate groups allow preconcentration of proteins with pI <4.25. The typical characteristics of the C matrix, such as reduced diffusion limitation for bigger molecules and higher flexibility of the polymer chains compared to polysaccharide-based hydrogels, are further advantages of these coatings.
Gelatin
Gelatin is an irreversibly hydrolyzed form of collagen with a broad molecular weight distribution of the polymer strands. Gelatin contains 19 amino acids, predominantly glycine, proline and hydroxyproline (>50%).
Hyaluronic acid (HY)
yaluronic acid is a strongly hydrated, anionic, nonsulfated glycosaminoglycan, which makes it unique among glycosaminoglycans as they are usually sulfated. The molecular weight of the hyaluronic acid used for the HY coatings lies between 2 and 4 MDa, which corresponds to 15,000–25,000 disaccharide monomers. The hyaluronic acid used is of animal origin (chicken).
Hyaluronic acid is a polymer of disaccharides, themselves composed of D-glucuronic acid and N-acetyl-D-glucosamine, linked via alternating β-(1→4) and β-(1→3) glycosidic bonds. Due to its high biocompatibility and common presence in the extracellular matrix of tissues, hyaluronic acid has been used as the basis for biomaterial scaffolds in tissue engineering.
As hyaluronic acid (HY) hydrogels are bioinert they can be a good choice for use in experiments with ligands/analytes that are prone to nonspecific interactions with CMD and HC surfaces. Further, they can be useful for biocompatibility research and tissue engineering.
Due to the abundant carboxyl groups, HY coatings are fully compatible with all immobilization chemistries available for CM-Dextran sensor chips.
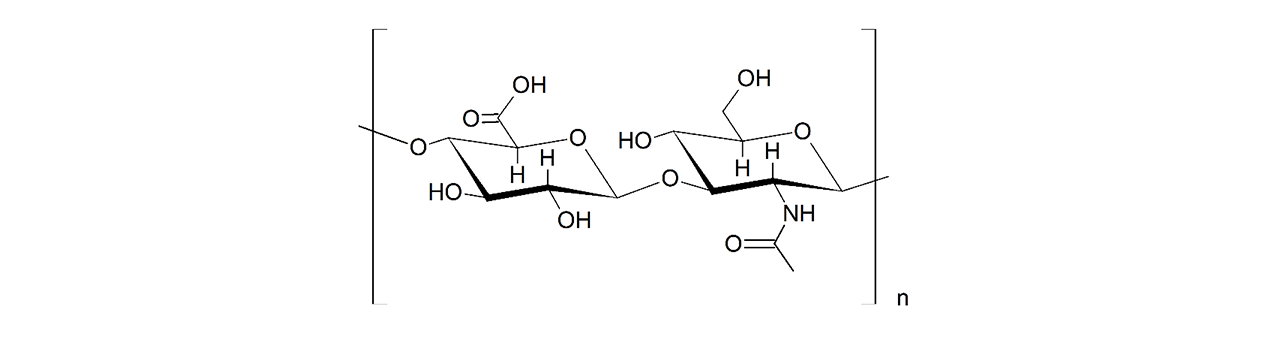
Hydrazide modified linear polycarboxylate (HZHC)
Based on the linear polycarboxylate HC with its superior biocompatibility and diffusion properties, the hydrazide modification of this surface coating offers mild and covalent immobilization of oxidized (poly)saccharides, antibodies through their oxidized carbohydrate side chains, or other molecules with aldehyde or ketone functional groups. The resulting hydrazone bond is stable if formed from ketones; if an aldehyde is used, it is recommended to stabilize the double bond by reduction.
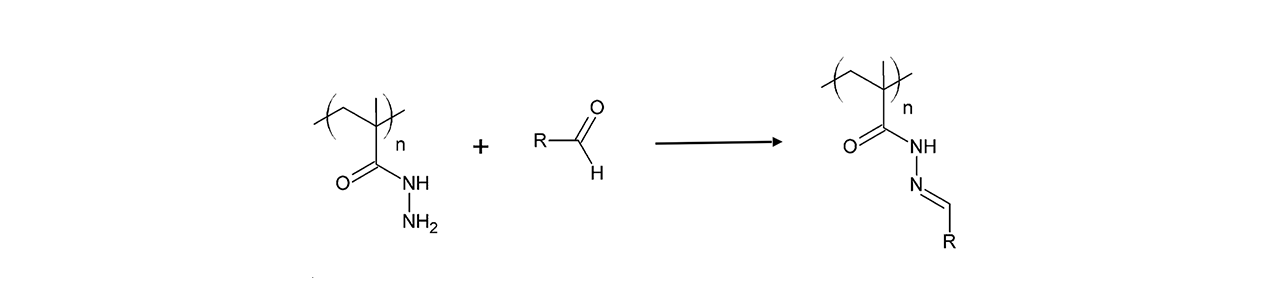
Pectin (PC)
Pectins, also known as pectic polysaccharides, are structural heteropolysaccharides contained in the primary cell walls of terrestrial plants. Isolated pectin, which is rich in galacturonic acid, has a typical molecular weight of 60,000–100,000 Da, varying according to the extraction conditions and origin.
Pectin hydrogels are useful chip surfaces with low nonspecific background for the analysis of biomolecules expressed in plants.
Due to the abundant carboxyl groups, PC coatings are fully compatible with all immobilization chemistries available for CM-Dextran sensor chips.
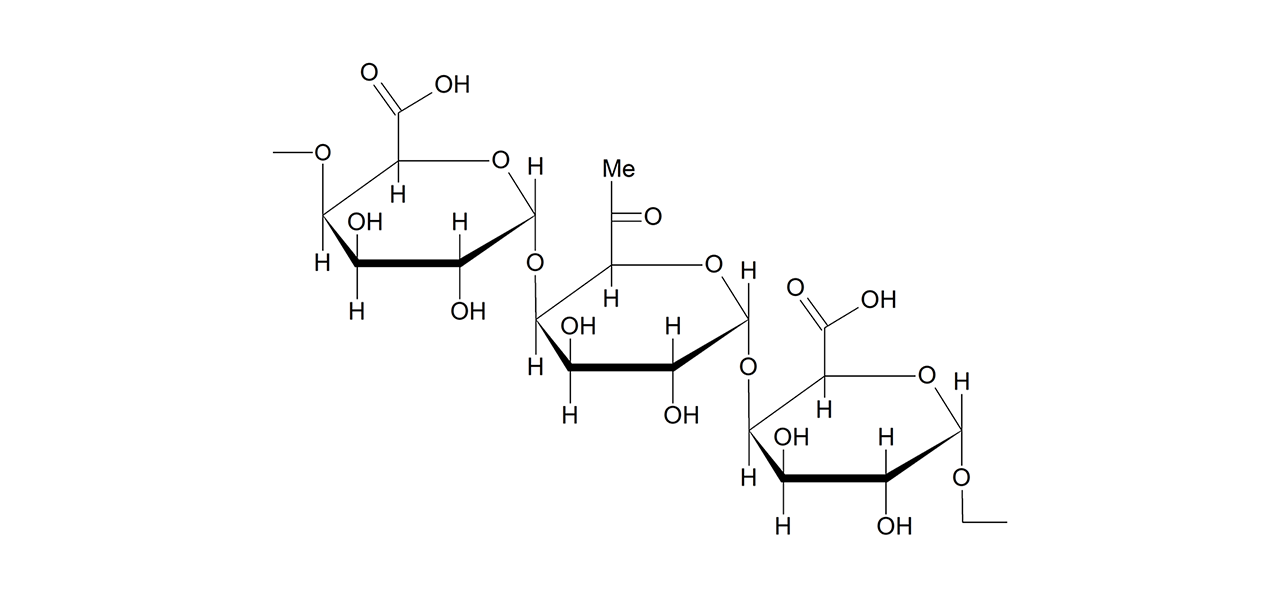
Polyethylene glycol (PEG)
The PEG sensor surface coating is a hydrophilic, polar surface based on a dense brush of 4-kDa PEG. The low polymer–water interfacial energy value results in good resistance to protein adsorption and cell adhesion.
The PEG surface is hydroxyl terminated and uncharged. It can be activated by oxidation to aldehydes and subsequent Schiff base coupling/reductive amination, or by (bis)epoxy activation. As there will not be any electrostatic preconcentration, high ligand concentrations are necessary to reach a sufficient level of immobilization.
Due to the terminal functionalization of the PEG chains, this surface is useful for the preparation of ligand monolayers on a well-stabilized, semi-hydrated and passive spacer layer.
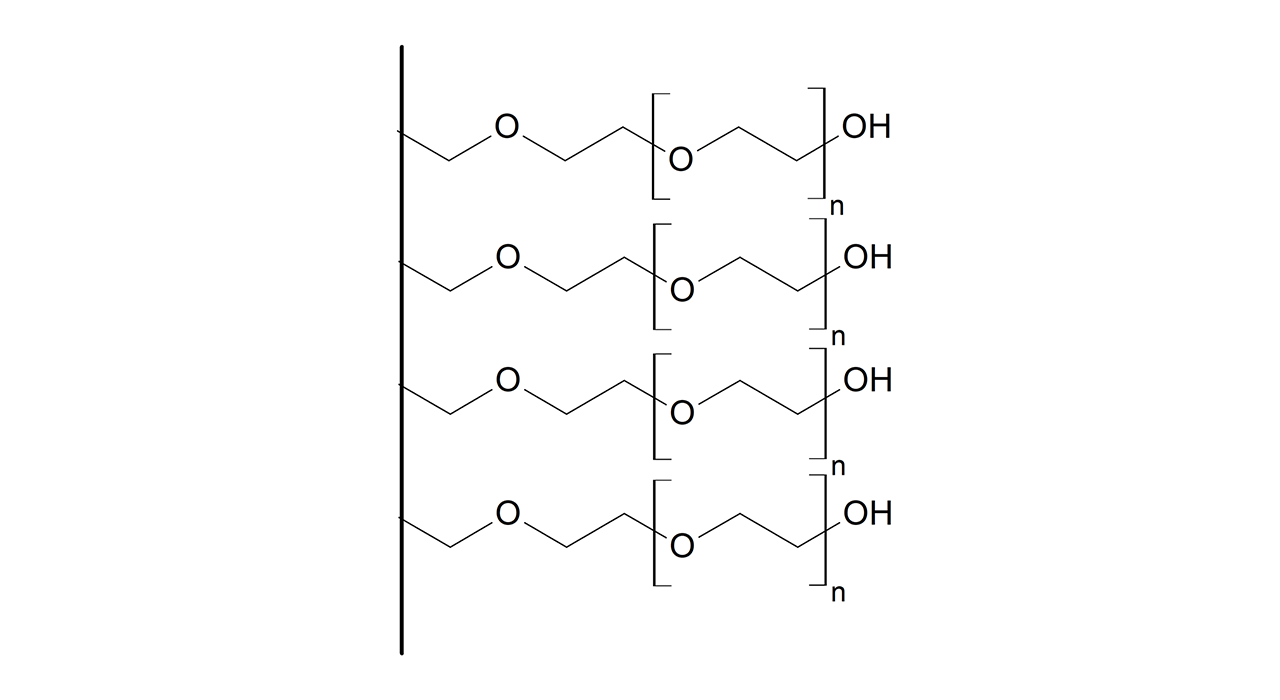
Tetraethylene glycol (TEG)
Tetraethylene glycol (TEG) is a member of a homologous series of dihydroxy alcohols and is well known for its biocompatibility. The sensor chip coating consists of a TEG self-assembled monolayer (SAM), which exclusively presents hydroxyl groups on the surface. Sensor chips with TEG SAMs have the advantage that the interactions take place very close to the gold surface, and thus in the most sensitive part of the evanescent field, resulting in acceptable SPR signals with medium-weight molecules as analytes. The TEG surface is ideal for the development of biocompatible surfaces, and can be used as a reference because of its well-documented ability to suppress protein adsorption and plasma activation. Furthermore, TEG can be modified chemically to alter the properties of the surface1, 2. Use of modified TEG sensor chips is of interest in the pharmaceutical and cosmetics industries, as TEG is often used as an additive in their products.
For TEG-based sensor chips with some carboxyl groups on the surface, which allow easy and gentle immobilization via EDC/NHS chemistry, please select CMTEG sensor chips.
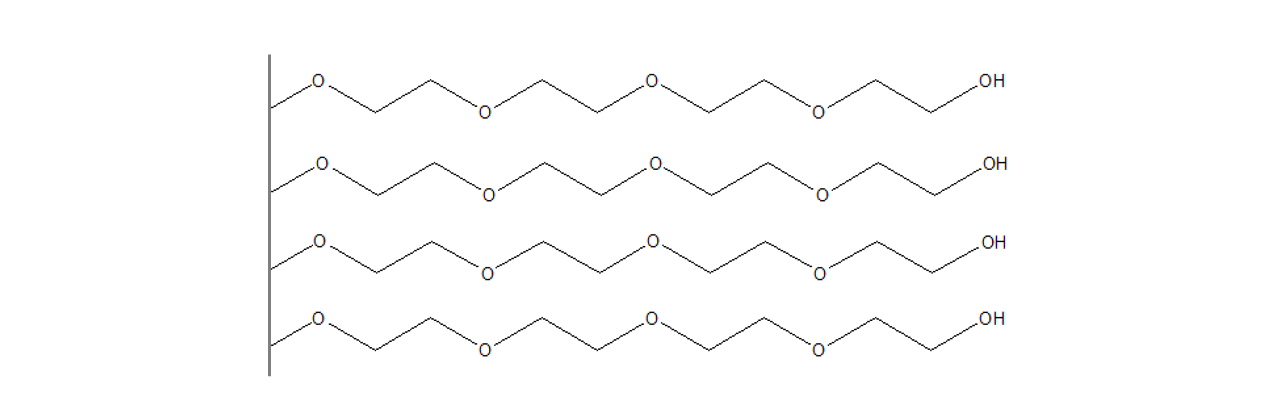
- Cha, T., Guo, A., Jun, Y., Pei, D., & Zhu, X. Y. (2004). Immobilization of oriented protein molecules on poly (ethylene glycol)‐coated Si (111). Proteomics, 4, 1965–1976.
- Piehler, J., Brecht, A., Valiokas, R., Liedberg, B., & Gauglitz, G. (2000). A high-density poly (ethylene glycol) polymer brush for immobilization on glass-type surfaces. Biosensors and Bioelectronics, 15, 473–481.
Disulfide modified hydrogels (THC, TD, TP)
THC, TD and TP sensor chips are coated with a disulfide derivatized bioinert hydrogel respectively 2D coating based either on carboxymethyldextran or the linear polycarboxylate HC. Covalent disulfide coupling requires reduction of surface and ligand disulfides and activation of the formed surface thiol groups with pyridyl disulfide before ligand injection. All THC, TD and TP coatings also contain carboxyl groups for electrostatic preconcentration of protein ligands.