95 Daltons and 201 Daltons Analyte Surface Plasmon Resonance (SPR) Analyses Using the 2SPR and XanTec CMD500m sensor chips
This Application note showcases both the very low noise of the 2SPR Dual Channel SPR system and the utility of XanTec's carboxymethyl dextran sensor chip, CMD500m, for use with small molecule interactions. The interaction between two small molecule inhibitors, methanesulfonamide (95 daltons) and 4-carboxybenzenesulfonamide (4-CBS) (201.2 daltons), and the enzyme carbonic anhydrase (CA II) is studied. Since carboxymethyl dextran sensor chips were first introduced into the marketplace about 20 years ago, they have become the industry standard for obtaining SPR data. They are used to reduce non-specific binding and increase sensitivity. The capture layer of the XanTec CMD500m sensor chip (catalog number SC CMD500m) is a 500 kDa carboxymethylated dextran hydrogel coupled proprietary grafting layer with superior characteristics. The combination of the 2SPR System and the XanTec CMD 500 m sensor chip yields excellent results for these interactions.
Experimental
The experimental conditions are summarized in the following table:
- Ligand: CAII
- Analyte: Methanesulfonamide
- Analyte Concentrations: 1066, 533, 266, 133, 66.6 μM
- Association Time: 1 min
- Dissociation Time: 1 min
- Analyte: 4-CBS
- Analyte Concentrations: 5, 2.5, 1.25, 0.625, 0.3125, 0.156 μM
- Association Time: 1 min
- Dissociation Time: 1.5 min
Results
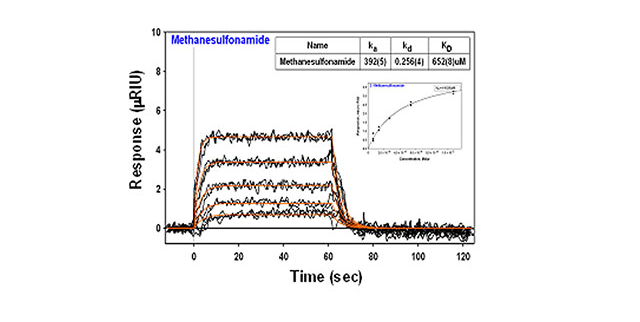
About 6,000 mRIU of carbonic anhydrase II was amine coupled to the dextran surface and then the binding of each inhibitor was followed over a series of concentrations (see table).The binding of the low molecular weight inhibitor, methansulfonamide, generates small but very detectable responses owing to the high sensitivity of the 2SPR. The kinetic fit from Scrubber (Biologic Software) yields an equilibrium dissociation constant (KD) of 652 mM, while the Langmuir isotherm (inset) yields a KD of 410 mM. Both values are within the range of values previously published by other researchers.
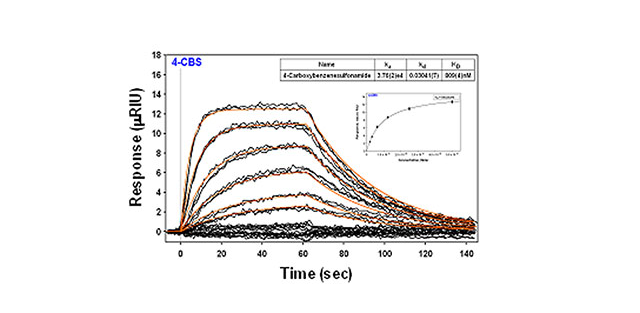
4-carboxybenzenesulfonamide binding to carbonic anhydrase II for a series of concentrations (see table) is shown here. Each concentration was injected at least in duplicate. The values obtained for the equilibrium dissociation constant (KD) are 809 nM for the kinetic fit from Scrubber (Biologic Software) and 940 nM for the Langmuir isotherm (inset). Both are within the range of values published in the literature for this binding pair using SPR and isothermal titration calorimetry (ITC).