Cancer researchers continue to work diligently to develop improved ways to deliver chemotherapies so that there are fewer side effects for patients. One intriguing way to do this is to use nanotechnology for the targeted delivery of drugs. This mode of treatment can lead to improvements in retention time in tumors as well as improved targeting. In the current study, researchers used a 2-channel Reichert SPR to study how peptide amphiphiles synthesized in their lab bind to a known cancer target and how these compounds could potentially be used in combination with the anti-cancer drug paclitaxel to provide improved outcomes.1
In drug development, researchers not only need to design the correct drug for the appropriate target molecule but must also determine the best delivery strategy. For this research, death receptor 5 (DR5), which is highly expressed in cancer cells and in metastasis or spread to other organs or tissues, was the target for cancer drug development. Researchers decided to develop a novel delivery vehicle of TRAIL-mimetic peptide-based nanostructures. The tumor necrosis factor family ligand for DR5 receptor, tumor necrosis factor related apoptosis-inducing ligand (TRAIL), is a unique endogenous molecule that initiates apoptosis in malignant cells in a preferential manner. Peptide based nanostructures have the added benefits of being less costly and having less stability issues than if the nanostructures are based on proteins or antibodies. The drug they used is a well-known anti-cancer drug paclitaxel, which is hydrophobic, and has been shown previously to enhance the pro-apoptotic effects of TRAIL receptor-targeted therapies.1
Experimental
Background
Nanostructures can be employed to make a cancer therapy more targeted, resulting in improved cytotoxicity of malignant cells but with less toxicity to the individual to whom the anti-cancer treatment is being administered. This study uses nanostructures which induce apoptosis on their own and become more potent in combination with the drug paclitaxel.
Conditions
- Instrument: Reichert SR7500DC
- Sensor Chip: Carboxymethyl Dextran
- Temperature: 25°C
- Target: Human Recombinant Death Receptor 5 (DR5)
(2000 µRIU immobilized) - Analyte: peptide or peptide amphiphile (PA) with and without paclitaxel
- Running Buffer: 10 mM phosphate, 150 mM NaCl, 3 mM EDTA, and 0.01 v/v% Tween 20, pH 7.0
- Flow Rate: 30 µL/min
- Association Time: 8 minutes
- Dissociation Time: 10 minutes Regeneration: 25 mM HCl for 30 seconds
Results
Researchers studied peptide amphiphile samples, with and without addition of the anti-cancer drug paclitaxel. These nanostructures contain TRAIL- mimetic peptides which are polyvalent in nature and can be combined and generate supramolecular self-assembly, meaning covalent bonds are not formed.1
SPR sensorgrams below show binding to DR5 of peptide amphiphiles alone and in combination with paclitaxel. The sensorgrams in Figure 1 show that co-assembly has only a minor effect on kinetics with calculated KD values of 2.4 nM for PA1 and 2.2 nM for co-assembled PA1 and PA4, respectively. Figure 2 results show binding kinetics with paclitaxel. This combination inhibited mammary tumor growth.1
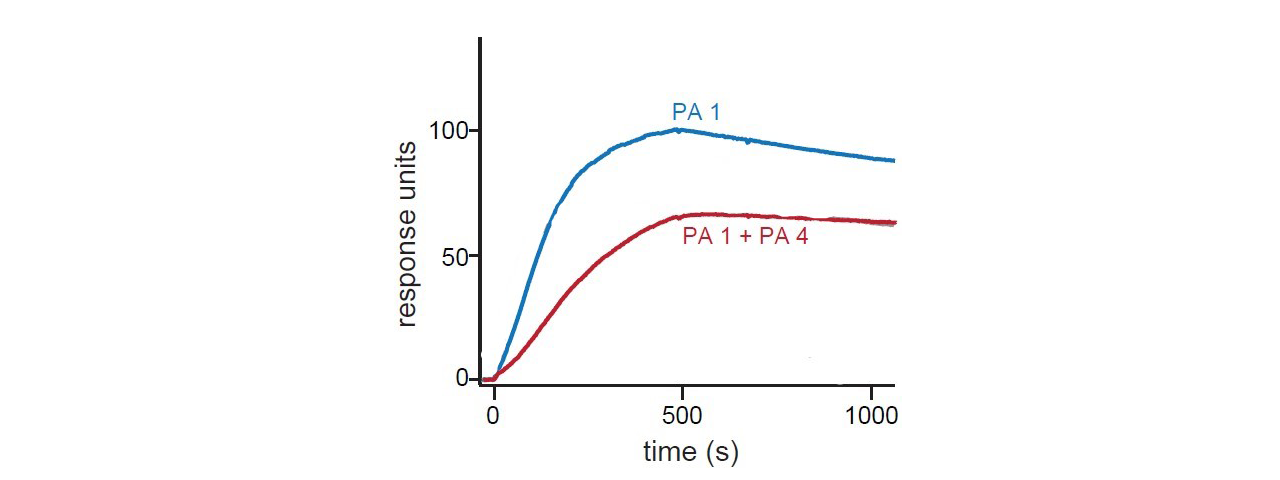
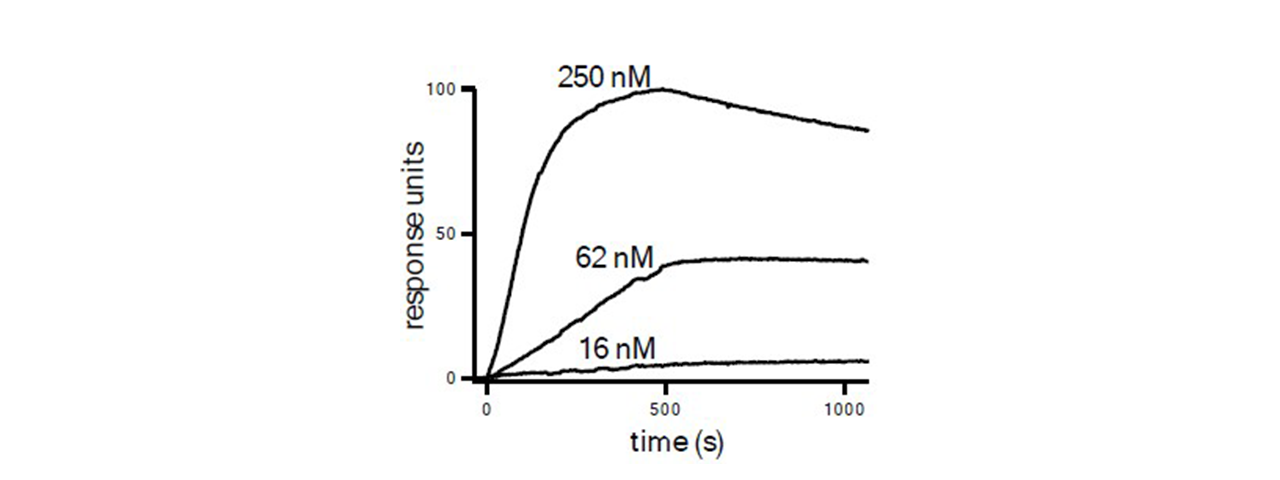
Summary
Peptide-based nanostructures can be used to induce apoptosis of tumor cells. In this work, a dimeric bioactive peptide amphiphile was synthesized which has high affinity for the DR5 death receptor. Each amphiphile can co-assemble with other peptide amphiphiles into supramolecular nanostructures. These nanostructures can form a hydrophobic compartment to encapsulate a chemotherapy drug so it can reach its target without breaking down or acting on other potential targets in the body. Results from this study indicate that it may be possible to make personalized nanomedicines which are rationally designed and incorporate therapeutic agents tailored to the particular molecular characteristics of the tumor.1
References
- Tyson J. Moyer, Feng Chen, Daniel J. Toft, Yves Ruff, Vincent L. Cryn and Samuel I. Stupp, “Self- Assembled Peptide Nanostructures Targeting Death Receptor 5 and Encapsulating Paclitaxel as a Multifunctional Cancer Therapy,” ACS Biomater. Sci. Eng., 2019, 5, 11, 6046-6053, DOI: 10.1021/acsbiomaterials.9b01259.
- Reprinted (Adapted) with permission from ACS Biomater. Sci. Eng., 2019, 5, 11, 6046–6053, Publication Date: October 23, 2019 https://doi.org/10.1021/acsbiomaterials.9b012 59, Copyright © 2019 American Chemical Society.