Researchers at the University of Arizona are studying Protein Phosphatase 1 (PP1), an enzyme involved in over 50% of all dephosphorylation reactions in the human body. In this current study, researchers sought to better understand how the known regulatory protein SDS22 interacts with PP1, including how it can act as both an activator and an inhibitor of PP11. Researchers used a number of techniques, including Surface Plasmon Resonance (SPR), to determine how different conformations of PP1 affect its binding to SDS22. In particular, they used their Reichert 4SPR to help them better understand the role that metals play in the binding interaction.
Protein Phosphatase 1 (PP1) is a Ser/Thr Phosphoprotein Phosphatase (PPP) that is abundant in many living organisms, including humans. PP1 is a single domain enzyme. It can form holoenzymes (combinations of enzymes with coenzymes that are biochemically active) with > 200 regulatory proteins. Previous research has shown that the active sites of all PPPs bind 2 metal ions (M1 and M2) and that the identity of the metals is dependent on the host.1 For instance, in mammals, the M2 metal is Fe+2 and the M1 metal is Zn+2. In bacteria, M1 and M2 are both Mn+2.
One of the regulatory proteins of PP1 is SDS22, which has long been known, but whose interaction with PP1 has not been fully understood. In this work, cellular, biophysical, and crystallographic studies were used to provide more information about the PP1:SDS22 interaction. Using crystallography, researchers were able to show that SDS22 binds to a form of PP1 that was not known previously and in addition, they found out more about how it acts as an inhibitor. With SPR they were able to expand their knowledge of how the binding is carried out, including information on the kinetics of the interaction.
Experimental
Background
Researchers were interested in better understanding the role of SDS22 in relation to PP1. They also wanted to determine what role the PP1 metals (M1 and M2) play in the PP1:SDS22 interaction.
Conditions
- Instrument: Reichert 4SPR
- Sensor Chip: Ni-NTA functionalized dextran (NiD50L; Xantec)
- Temperature: 25°C
- Targets: PP1 variants
- Analytes: SDS22, Control NIPP1
- Buffer: 20 mM Tris pH 8.0, 500 mM NaCl, 0.5 mM TCEP, 0.05% Tween (with and without the addition of 1 mM MnCl2 or 1 mM ZnCl2)
- Association: 1.5 minutes
- Dissociation: 3 minutes
- Flow Rate: 50 μL/min
- Regeneration: 350 mM EDTA pH 8, 10 mM NaOH
- Recharge surface: 40 mM NiSO4
Results
Using multiple techniques, researchers determined that SDS22 binds exclusively to metal deficient PP1 (PP1 with M2 only). To determine the mechanism, ie. whether SDS22 causes the loss of metal at the active site or binds PP1 after the metal has been lost, they used crystallography and surface plasmon resonance (SPR). Results indicated that SDS22 binds PP1 after the metal is lost.1
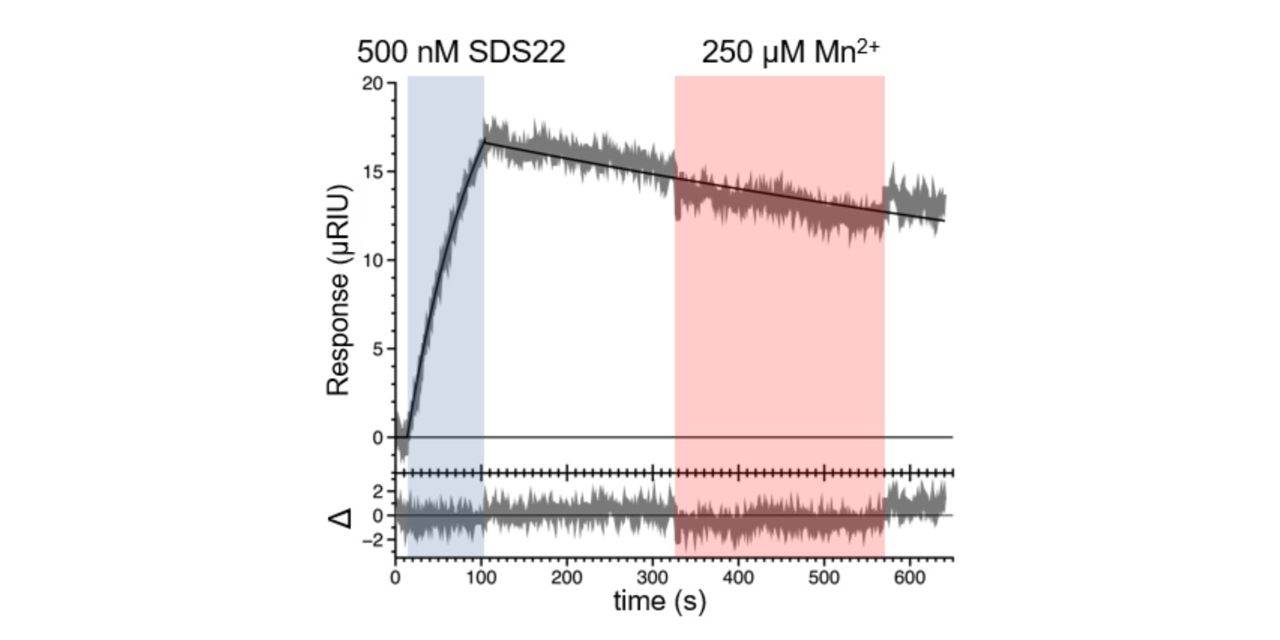
Researchers also tried adding metal into the buffer at the dissociation step of the PP1:SDS22 binding interaction. They found that the dissociation was not affected (Figure 2), indicating that the addition of metal alone does not break up the interaction.
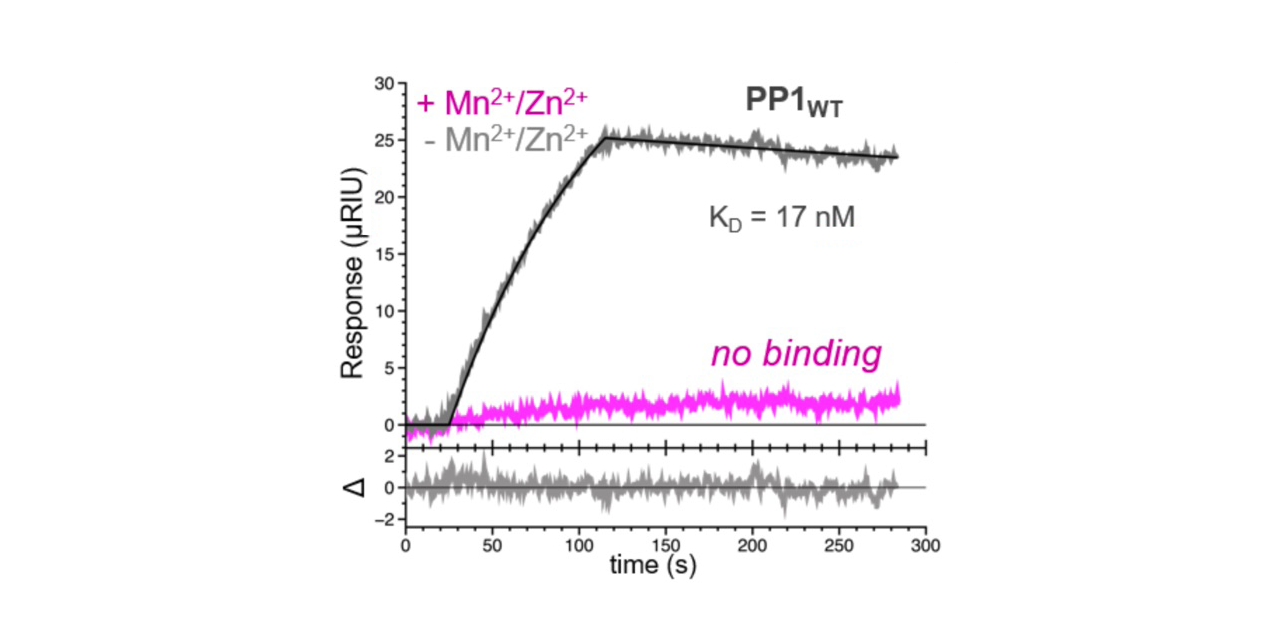
SPR experimental results showed that the interaction between SDS22 and PP1 lacking M1 is tight, with a KD of 16.9 ± 5.1 nM. While this value is similar to that obtained for other PP1 regulators, the interaction is not typical in that the association (on) and dissociation (off) rates are both slower. Along with data obtained using other techniques, researchers were able to conclude that the slower off rate helps SDS22 stabilize and trap the inactive metal-deficient conformation of PP11.
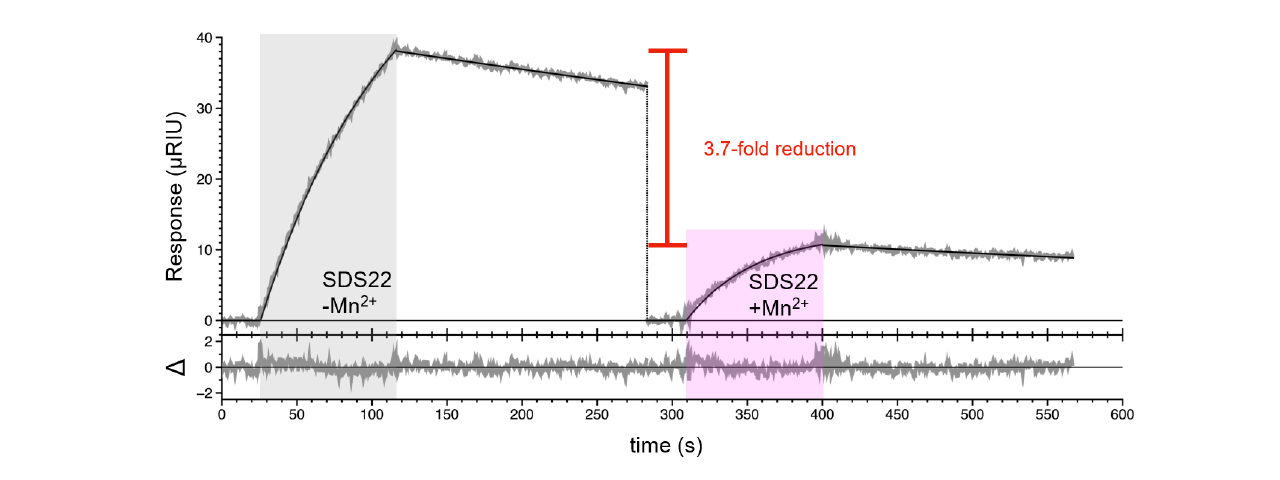
There was an additional question whether Mn+2 and SDS22 compete for binding to PP1. It was confirmed they do with approximately a 3.7 fold reduction in SDS22 binding in buffer containing Mn+2 (Figure 3).
Summary
- The key finding from this study is that researchers determined that SDS22 binds a previously unknown conformation of PP1 that contains a single metal (M2 only, not M1) at its active site. The PP1 with only one metal is inactive, so SDS22 is effectively trapping metal-deficient (inactive) PP1.
- The mechanism of the binding was also determined – ie. data was obtained that confirms that SDS22 binding does not cause removal of the M1 metal from the PP1 active site, but instead, selectively binds PP1 that is lacking M1 metal at the active site (has M2 only).
- The dissociation of the complex is slow, indicating that SDS22 forms a tight, long-lived complex with PP1. In addition, when SDS22 dissociates, M1 metal is loaded into PP1. Hence, SDS22 provides a"pool" of inactive PP1 ready for holoenzyme formation when needed and it simultaneously prevents unregulated PP1 activity in the cell.
Reference
- Meng S. Choy, Thomas M. Moon, Rini Ravindran, Johnny A. Bray, Lucy C. Robinson, Tara L. Archuleta, Wuxian Shi, Wolfgang Peti, Kelly Tatchell, Rebecca Page, “SDS22 selectively recognizes and traps metal-deficient inactive PP1,” Proceedings of the National Academy of Sciences, Sep 2019, 201908718.