Research at SUNY Buffalo State involving the study of mass transport across lipid bilayers mediated by channel proteins and transporters is being carried out using Reichert SPR instrumentation. These experiments require confidence in the ability to anchor intact liposomes to the sensor surface without the undesired formation of lipid monolayers or fused tethered bilayers. To gain a better understanding of how lipids deposit on different SPR sensor surfaces, researchers conducted some preliminary studies using capture levels, relative protein-lipid binding capacity, and off-line fluorescence microscopy to help distinguish between the different possible modes of lipid deposition.
The goal of the work was to determine how lipid vesicles interact with different types of surfaces and to identify conditions needed to establish a stable layer of intact liposomes. Three types of surfaces were compared: a hydrophobic planar alkanethiol surface (Reichert’s HPP surface), a planar NeutrAvidin surface (Reichert’s NeutrAvidin surface), and a Phytosphingosine surface prepared on a planar carboxylated surface (Reichert’s COOH surface). NeutrAvidin was used as an anchor to capture vesicles that include a small percentage of a lipid containing a biotin-modified headgroup. Alternatively, Phytosphingosine was used to anchor the same vesicles directly by membrane insertion and thus has the advantage of not requiring the inclusion of specific modified lipids during liposome preparation. Several lines of evidence suggest that liposomes fuse and rearrange into a monolayer on the HPP surfaces whereas liposomes remain intact when tethered to a COOH surface through either a NeutrAvidin or Phytosphingosine anchor.
Experimental
Background
Small unilamellar vesicles containing Egg PC and PE-Biotinyl CAP at a 99:1 mole ratio were prepared by extrusion through a 100 nm pore membrane at 50°C and diluted in PBS to approximately 10 mM lipid prior to injection over the sample channel of different SPR surfaces. The inclusion of a biotinylated lipid during vesicle preparation served two different purposes. One, it was used to capture vesicles specifically on the NeutrAvidin surface. Two, it was used to serve as a lipid ligand for the binding of NeutrAvidin analyte injected over the surface. The same vesicle preparation was used on different surfaces to ensure that the biotintylated lipid ligand was a constant fraction of total lipids across all experiments. The ratio of NeutrAvidin bound to total captured lipids would be expected to be half as high for intact vesicles compared to lipid monolayers because the inner membrane leaflet of the vesicle contributes roughly half the total lipid mass but is unavailable for binding. Inclusion of a green fluorescent polar dye (Calcein) and a red fluorescent lipophilic dye (Rhodamine-PE or DiI) during vesicle preparation was used to determine if liposomes remained intact on different surfaces.
Conditions
- Instrument: SR7500DC (now called a Reichert2SPR)
- Sensor Chips: Planar Hydrophobic Alkanethiol (HPP), Planar PEG/Carboxyl (COOH), Planar NeutrAvidin
- Running Buffer: PBS
- Flow Rate: variable, 5 μl/min (vesicle capture), 30 μl/min (BSA and NeutrAvidin), 50-100 μl/min (NaOH washes)
- Temperature: 25°C
- Vesicle Composition: Egg PC, PE-Biotinyl Cap (99:1 mole ratio) Regeneration Buffer: 20 mM CHAPS
- HPP Surface precondition: 40 mM d-octylglucoside
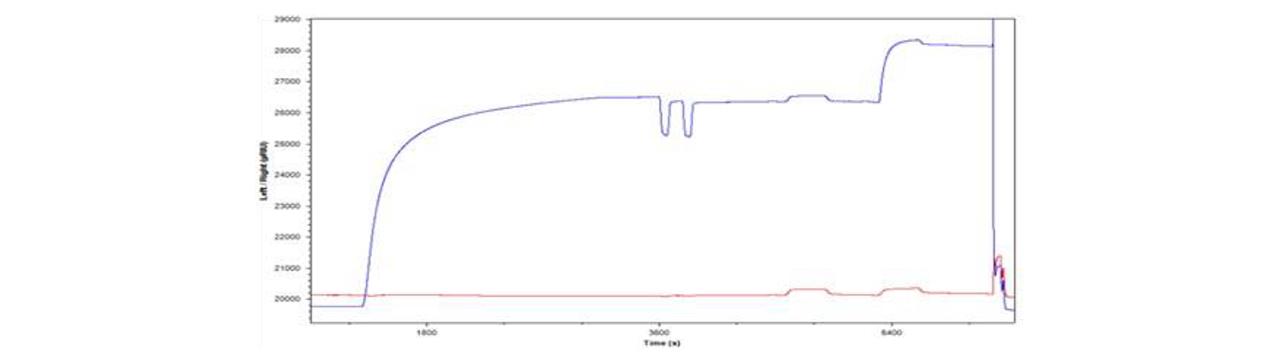
Results
A standard protocol, shown in Figure 1, was developed to reproducibly load vesicles onto each sensor chip, assess and block non-specific binding sites with BSA and then quantify specific binding of NeutrAvidin to the biotintylated lipid groups, followed by removing lipid with CHAPS to regenerate the surface for another cycle. Lipid capture levels were reproducible on NeutrAvidin and Phytosphingosine surfaces over many cycles, but not on HPP surfaces (data not shown).
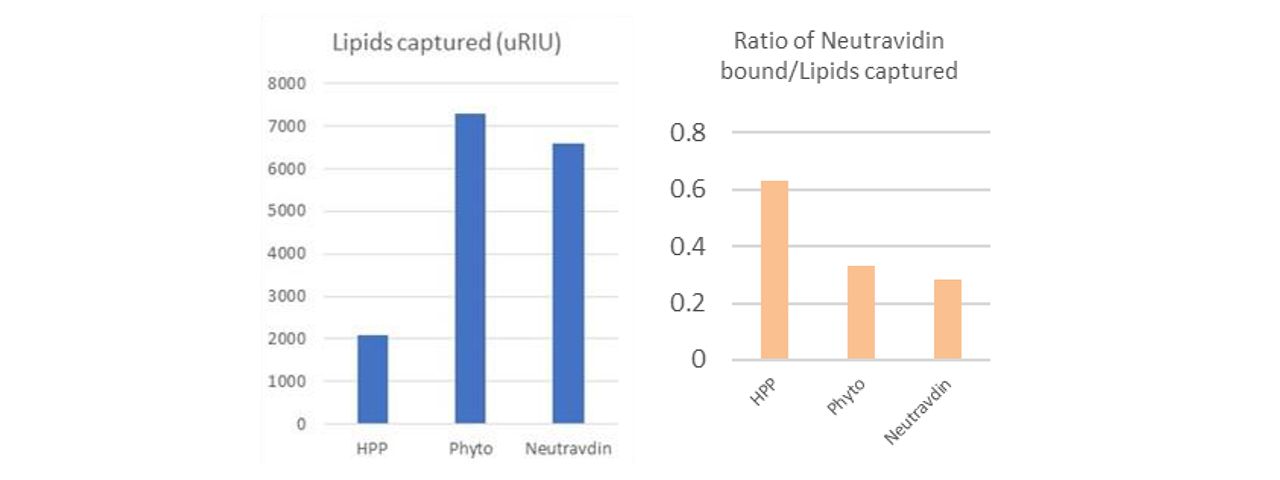
Typical lipid capture levels and NeutrAvidin analyte binding relative to total lipids for each of the three types of surfaces is provided in Figure 2. HPP surfaces capture significantly less lipid mass upon exposure to liposomes compared to Phytosphingosine or NeutrAvidin surfaces, consistent with the idea that lipids are deposited as a monolayer on the HPP surfaces rather than intact vesicles (Figure 2, left panel). Supporting this idea is the fact that the lipid coated HPP surfaces bind 2-fold more NeutrAvidin analyte relative to total captured lipid mass compared to the NeutrAvidin and Phytosphingosine surfaces (Figure 2, right panel). Overall, findings indicate that the liposomes are deposited as bilayer structures on the latter two surfaces wherein half of the total lipid mass (the inner membrane leaflet) is unavailable for binding a membrane impermeable analyte such as NeutrAvidin.
Fluorescence was used to study how the liposomes deposited on the 3 different sensor chip surfaces (Figure 3). Liposomes with a red fluorescent lipid label (Rhodamine-PE at 0.5% mole ratio) and encapsulating a polar green fluorescent dye (1 mM Calcein) were deposited on each of the surfaces and then viewed by fluorescence microscopy. Exposure to air destroyed the lipid coating on HPP surfaces so an HPP and a NeutrAvidin surface (for direct comparison) were processed offline in petri dishes applying the same protocol described above but using gentle rocking to generate flow across the surface. The Phytosphingosine surface was removed directly from the instrument for imaging and shows a curved region of the capture channel. The absence of entrapped green fluorescence on the HPP surface suggests fusion of the vesicles with the surface.
| Surface | (A) Phase Contrast | (B) Red Lipid Fluorescence | (C) Green Polar Tracer |
|---|---|---|---|
| HPP |  |
 |
 |
| Neutravidin |  |
 |
 |
| Phytosphingosine |  |
 |
Summary
- Liposomes can be captured and remain intact on COOH surfaces using a NeutrAvidin or Phytosphingosine anchor. The NeutrAvdin anchor requires a biotin group exposed on the liposomes whereas the Phytosphingosine anchor allows capture of liposomes of any composition.
- The behavior of lipid deposition on hydrophobic HPP surfaces suggests the fusion of liposomes with the surface and rearrangement of lipids into a monolayer.
- The determination of how lipids deposit on a surface (monolayer vs tethered bilayer vs intact vesicles) can be supported by a comparative analysis of analyte/lipid binding capacity and offline fluorescence microscopy techniques.
Conclusions
- Results of testing indicate that lipid vesicles remain intact on either the Phytosphingosine or NeutrAvidin surfaces but not on the HPP surface. The HPP will not be used going forward.
- Future work will proceed with Phytosphingosine surfaces because they do not require any special lipid tags to capture vesicles. These surfaces will be used to anchor natural vesicles harvested from membrane blebs induced in eukaryotic cells.