Introduction
Researchers at Penn State University have been studying aptamers for a number of years.1 For their current research, they combined aptamers with proteins to create macromolecules that can both assemble and maintain molecular recognition under physiological conditions. This application is particularly interesting since most macromolecules do not maintain their molecular recognition properties when they assemble. While proteins can assemble, the ability to maintain molecular recognition was expected to be made possible by the use of aptamers. In addition, these new macromers were studied to see if they would afford sustained release of VEGF to promote wound healing in mice. 2 Fibrinogen and an anti-vascular endothelial growth factor (VEGF) aptamer were used to synthesize a hybrid aptamer-fibrinogen macromer. Fibrinogen is a natural protein that can assemble to form fibrin hydrogels. Aptamers are single-stranded oligonucleotides with high affinities and specificities to target molecules. Aptamers can be selected from large libraries and form functional structures through high-fidelity base pairing. This intramolecular hybridization would not affect the functional conformation of the aptamer or protein in an aptamer-protein macromer. Thus, it has been hypothesized and then shown in this work that Fibrinogen-aptamer macromers both assemble and form Fibrin hydrogels and maintain their molecular recognition capability.2
A variety of analysis techniques were used by researchers to verify both the assembly and molecular recognition properties of the macromolecule they formed. The secondary structure of the macromer was studied with circular dichroism and the assembly was studied using electron microscopy. Surface plasmon resonance was the primary technique used to determine whether molecular recognition was maintained in the combined molecule and a Reichert SPR instrument was used to do the analysis. The aptamer-Fibrin hydrogels were further studied to determine their roles in controlling continuous VEGF release and promoting cell growth both in vitro and in vivo. VEGF is a potent angiogenic growth factor and the in vivo study was performed by studying the growth of new blood vessels for skin wound healing in mice.2
Experimental
Background for SPR Study
Vascular Endothelial Growth Factor (VEGF) was immobilized on a planar chip using standard amine coupling. After equilibration, different compounds were flowed over the surface including free Aptamers, native Fibrinogen, a Thiol-modified scrambled aptamers-fibrinogen macromer (used as a control), and the Aptamer-Fibrinogen macromer which was the focus of this study.
Conditions
- Instrument: SR7500DC/Reichert2SPR
- Sensor Chip: Planar
- Running Buffer: PBST
- Flow Rate: 25 μL/min
- Temperature: 25°C
- Target: VEGF
- Analytes: native Fibrinogen, free aptamers, Aptamer-Fibrinogen macromer and scrambled aptamers-Fibrinogen
Results
Researchers found that their Aptamer-Fibrinogen macromer showed the largest response of all the samples tested (even though the size is comparable to all the other samples except for the aptamer alone) and had a slightly lower affinity than the KD for the Aptamer alone binding to VEGF (they found a KD of 240 nM for the Aptamer-Fibrinogen macromer versus 160 nM for free aptamer – these values are considered to be of moderate affinity related to this type of research).2 Other samples that were tested (scrambled aptamer-fibrinogen control and fibrinogen alone) showed only minimal binding and are not included in the figure below.
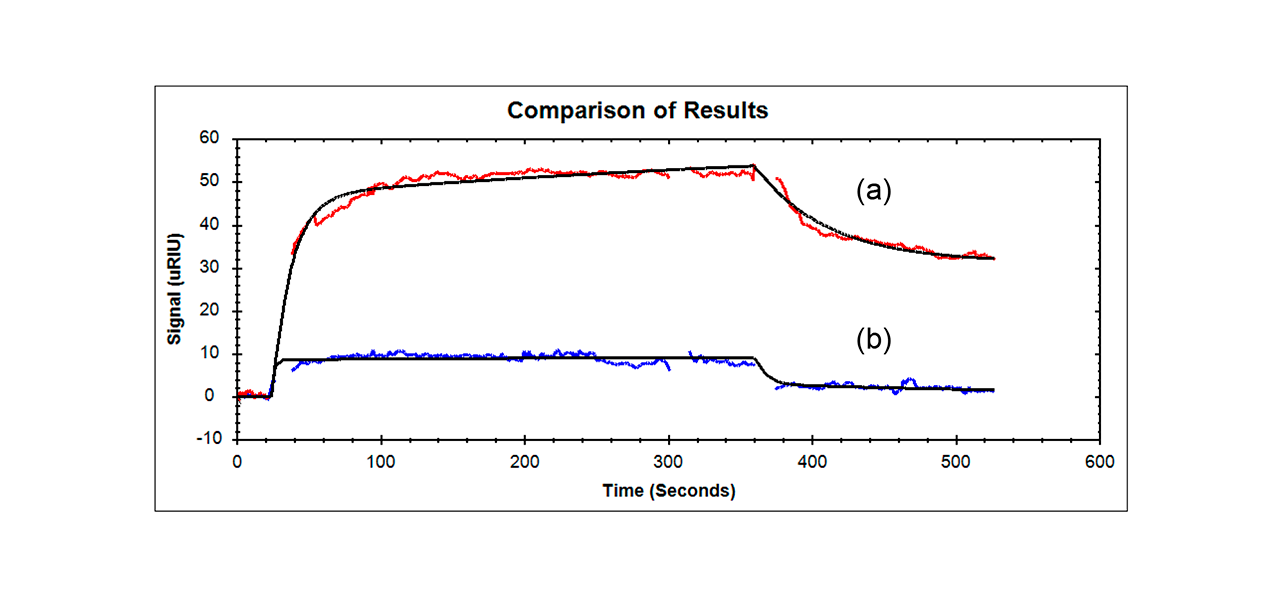
Summary
- An Anti-VEGF aptamer- Fibrinogen macromer bound to immobilized VEGF using SPR and its response had a similar affinity to aptamer alone. In addition, the Anti-VEGF aptamer- Fibrinogen macromer showed a 5-10 times larger response than aptamer alone, fibrinogen alone or scrambled aptamer-Fibrinogen even though all samples but the aptamer alone were about the same size.
- Reichert SPR results provided confirmation that molecular recognition was maintained for the aptamer-fibrinogen macromer.
- Results here are preliminary but the idea of combining an anti-VEGF aptamer with fibrinogen to form a macromer show promise in the fields of drug delivery and regenerative medicine. The Penn State macromer showed enhanced wound healing in mice due to the sustained release properties of the macromer hydrogel. Future research will involve the study and optimization of parameters. For instance, a higher affinity aptamer (perhaps 10- fold higher than the current KD (ca 10 nM)) should improve the release profile of the macromer hydrogel.2
References
- Penn State website: sites.psu.edu/yxwanglab/research/
- Nan Zhao, James Coyne, Ming Xu, Xiaolong Zhang, Akiho Suzuki, Peng Shi, Jinping Lai, Guo-Hua Fong, Na Xiong, and Yong Wang, "Assembly of Bifunctional Aptamer- Fibrinogen Macromer for VEGF Delivery and Skin Wound Healing," Chem. Mater., Just Accepted Manuscript o DOI: 10.1021/acs.chemmater.8b04486. Publication Date (Web): 11 Jan 2019.