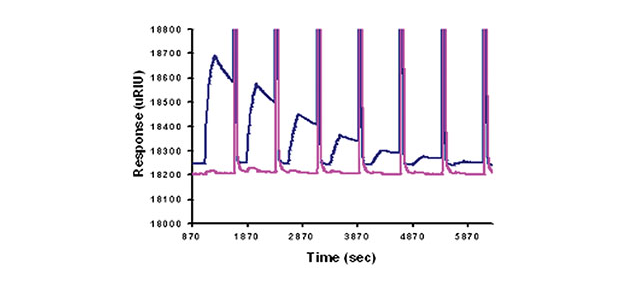
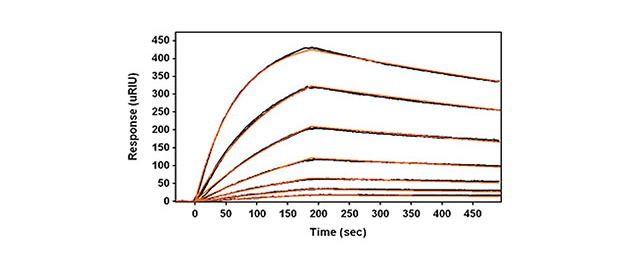
Human Serum Albumin (HSA) Binding to Biotinylated Anti-HSA lgG Captured on Planar Neutravidin Surface
Human serum albumin (HSA) is a 67 kDa protein and is the most abundant protein in human blood serum. HSA is essential to the blood transport system as it is responsible for the transport of several physiologically important compounds such as fatty acids, bilirubin, calcium, steroid hormones, and various drugs. This Application note presents the binding kinetics of a model antibody-antigen system, HSA binding to anti-HSA IgG. Anti-HSA is biotinylated with approximately 6 biotin groups (Solulink, part no. B-1001-105) and captured on a planar neutrAvidin sensor slide.
Experimental
The experimental conditions for this assay are summarized below:
- Ligand: Anti-HSA
- Analyte: HSA
- Analyte Concentrations: 80,40,20,10,5,2.5,1.25 nM
- Association Time: 3 min
- Dissociation Time: 5 min
- Regeneration Solution: Cocktail
Results
The SR7000DC SPR Dual Channel System monitors this antibody-antigen interaction in real-time with simultaneous monitoring of sample and reference channels.