Biacore™, a spin-off from the Swedish biotech company Pharmacia©, launched the first commercial SPR biosensor in 1991. As Pharmacia© was a pioneer in dextran-based separation media (Sephadex™), it was obvious to simply transfer this technology to SPR sensor chips. In 1997, researchers at XanTec bioanalytics conducted systematic studies aiming to identify the optimal coating technology for optical biosensors. This work was successful, and led to our current position as the technology leader in high-performance sensor coatings.
Today XanTec bioanalytics is the leading original equipment manufacturer (OEM) of sophisticated nanostructured sensor surfaces for a number of SPR instruments. The company also supplies sensor chips directly to >1000 end-users, including most big pharma companies and top research institutions around the world. Our sensor chip portfolio is the biggest on the market and compatible with most instrument brands in use today. This unique offering enables SPR users to directly compare their results and transfer protocols between different instrument platforms.
Beside the ability to use the chips in different makes and model of instrument, one of the most frequently asked questions concerns the comparability of the surfaces with those of Biacore™ (Cytiva®) sensor chips, and the comparability of data generated using different instruments.
In the following, we’ll elaborate on technical aspects that are important for understanding the advantages of XanTec’s chips compared with the standard chip chemistry. Examples and comparative literature will be presented to illuminate central aspects and highlight specific characteristics of the various sensor chip types.
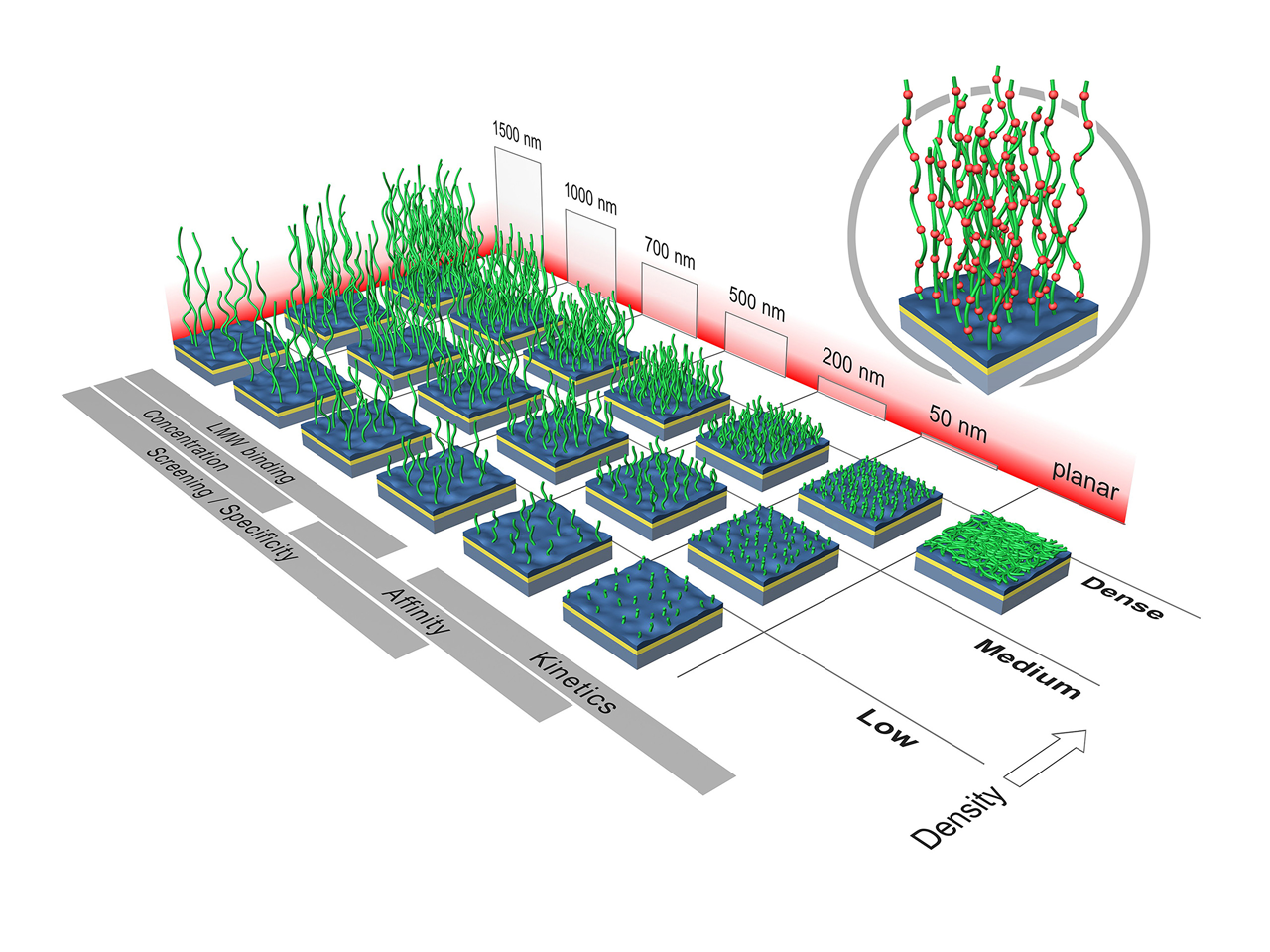
Probably the biggest difference between the sensor chip portfolios from XanTec and Biacore™ is that XanTec offers more structural variety: different thicknesses, different densities of the hydrogel, and different polymers are available. The resulting sensor chip matrix (Fig. 1) is an optimally tailored application-specific immobilization basis for all possible ligand–analyte combinations.
While the thickness of the hydrogel is largely determined by the application or the immobilization capacity to be achieved, the choice of the density of the hydrogel depends on the volume of the analyte molecule. In general, the smaller the analyte, the denser the hydrogel can be without steric effects becoming apparent. For analytes <5 kDa, denser hydrogels (M or D) are advantageous; for analytes up to approx. 100 kDa, medium polymer densities (M) are ideal; and for larger analytes, sensor chips with low-density polymer structures (L) or even 2D surfaces should be used to minimize diffusion limitation and steric hindrance.
In recent years, our sensor chip surfaces have frequently been compared with those of Biacore™, for example. This has been the case both in direct comparisons and in cross-platform comparative studies in both the pharmaceutical industry and academia.
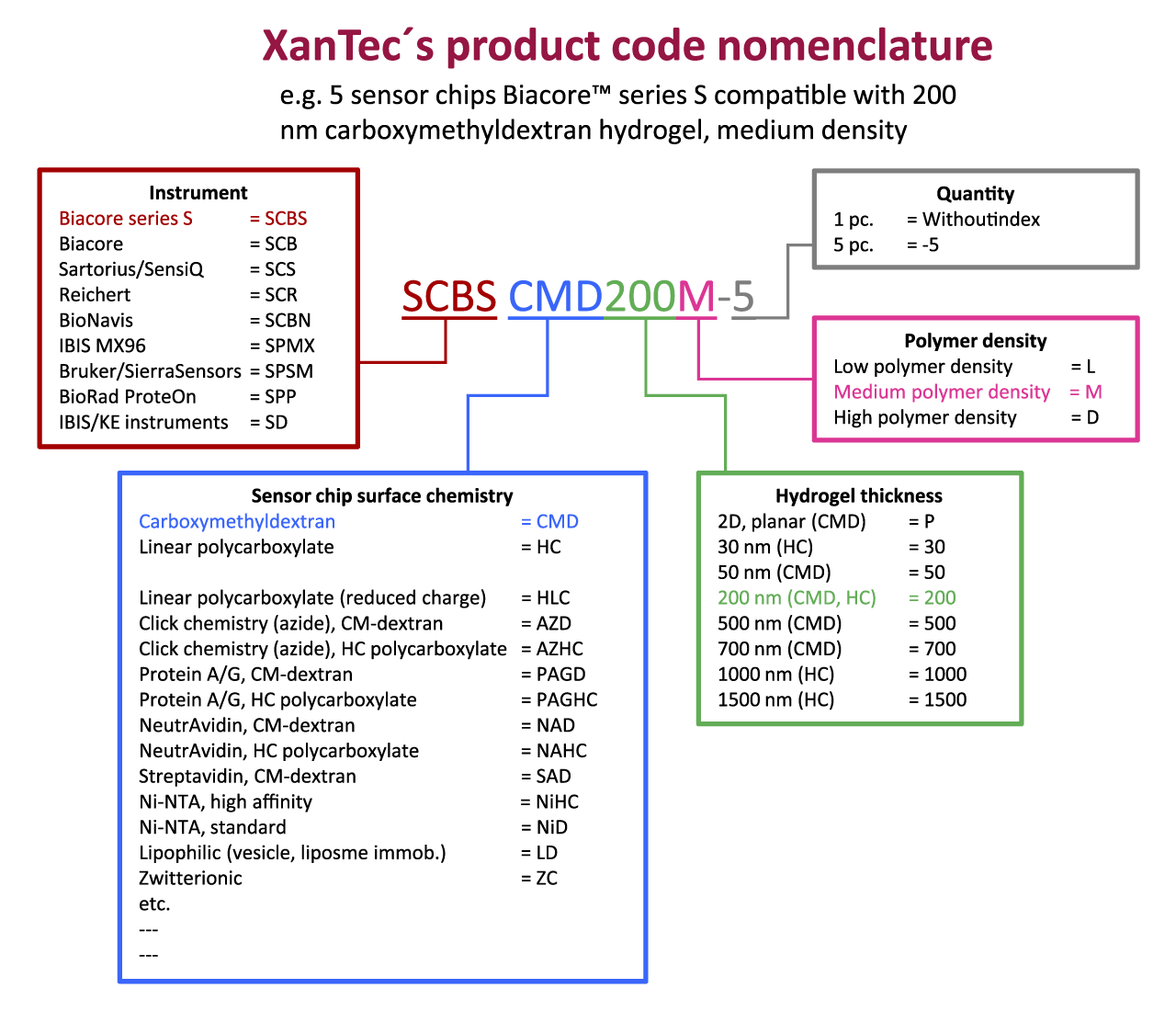
To note one aspect first: All independent studies confirmed that XanTec sensor chips perform equally to their Biacore™ equivalents in terms of immobilization capacity, suppression of nonspecific interactions, regeneration, and so on. However, it should be noted that such comparisons were based on the limited chip portfolio of Biacore™ and that XanTec’s significantly superior performing polymers such as the linear polycarboxylates HC and HLC were mostly not included in these studies, simply because Biacore™ cannot offer such coatings.

By far the most popular immobilization method for SPR is covalent attachment via EDC/NHS to carboxyl-functionalized sensor chip surfaces. Although Biacore™ has a small selection of sensor chips of this type with different immobilization capacities, most users rely on the CM5 version.
In an interesting study by Brown et al.1, the CM5 and C1 chips from Biacore™ were compared with their counterparts from XanTec, the CMD200M (Table 1) and the CMDP. C1 and CMDP are planar (2D) sensor chips, as the analytes were sterically challenging antibodies. The comparative measurements were carried out on a Biacore™ 8K instrument. The results are not surprising: The planar sensor chips from XanTec and Biacore™ as well as the 3D hydrogel chips (CMD200M and CM5) deliver almost identical results for the association/dissociation rate constants (ka & kd) and affinity (KD) (Table 1). However, XanTec’s planar CMDP chip had 4-times the immobilization capacity of the C1 chip from Biacore™, which significantly expands the application range towards smaller analytes that can still be analyzed without the need to use hydrogel coatings.
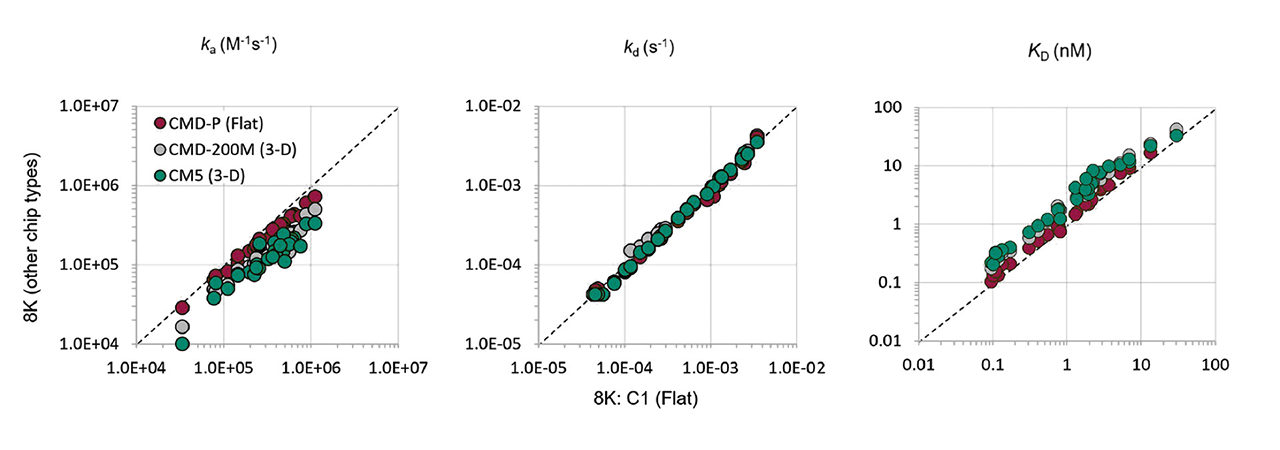
Experiments on the Biacore™ 8K instrument also showed that 3D-hydrogels (Biacore™ CM5 & XanTec CMD200M) produced systematically slower on-rates and weaker affinities than 2D coatings, with no discernible effect on the off-rate.
After intensive testing comparing XanTec’s CMD200M surface with the CM5 chip from Biacore™, a big pharmaceutical company concluded that the CMD200M chips were “recommended as a substitute.”6 Particular emphasis was placed on the “good immobilization results,” the “good biospecific interaction results,” the “stable baseline,” and the “complete matrix regeneration.” Furthermore, “no chip-related error messages were displayed by the BPU or Biacore™ Control Software.” Moreover, no problems occurred during docking/undocking of the Biacore™-instrument-compatible sensor chips and subsequent repeated insertions. The 3D sensor chips from XanTec (CMD200L/M & CMD500L) and Biacore™ (CM5) (Fig. 5) were also found to have very similar or even identical characteristics in other studies2, 3, 4, 5.
| Modification | Biacore™ | XanTec |
|---|---|---|
| Plain gold surface | Au | Au |
| No hydrogel, low capacity | C1 | CMDP / HCP |
| Short matrix, normal capacity | CM3 | CMD50L, HC30M |
| Standard hydrogel, low charge density | CM4 | HLC200M |
| Standard hydrogel, normal capacity | CM5 | CMD200M CMD500L, HC200M |
| Standard hydrogel, high capacity | CM7 | CMD700M, HC1500M |
| NTA for His6 tagged ligands | NTA | High affinity: NiHC1500M, NiHC200M Standard affinity: NiD200M |
| Hydrophobic 2D surface | HPA | HPP |
| Vesicle and liposome capture | L1 | LD (hydrogel) & LP (2D) |
| Biotin capturing surface | Streptavidin: SAD200M & others Neutravidin: NAHC200M & others |
|
| Click chemistry | Azide surface: AZHC200M & others DBCO surface: DCD200M & others |
|
| Zwitterionic surface | ZC30M, ZC80M, ZC150D | |
| Biotin derivatized surface | BHC30M, BD200M & others |
This compatibility is not surprising, as the hydrogel material of XanTec’s CMD coatings is practically identical to the carboxymethyldextran used on Biacore™ sensor chips. However, there are a few structural differences which result in improved performance:
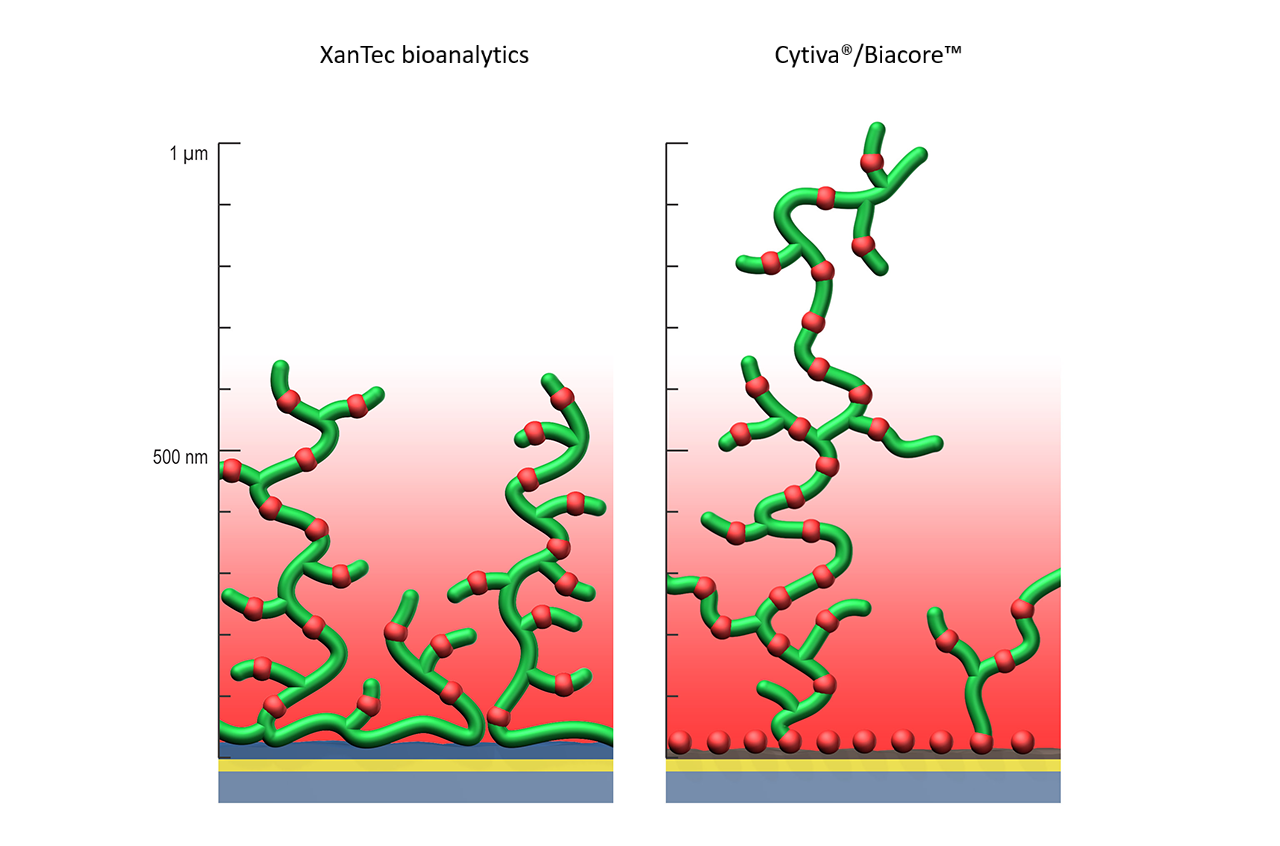
Left: XanTec’s homogenously distributed ligand coupling sites fully covered by the evanescent field (EF).
Right: Biacore™ self-assembled monolayer-based approach (brown) with a high density of negative charges and hydrophobic domains close to the gold surface, but the majority of the ligand population in the less sensitive outer region of the EF.
The basis for XanTec’s adaptive chip architecture is two-part. First, the use of a hydrophilic polymer adhesion promoter, which - unlike the self-assembled monolayer used by Biacore™ and others - covers atomic defects in the gold layer and shields the surface against non-specific interactions with hydrophobic sample components. Second, the optimized XanTec polymer surface structure concentrates the ligand binding sites in the lower, more sensitive region of the evanescent field. Negative charges in the vicinity of the gold film, which are critical for non-specific interactions (Fig. 5), are eliminated. Both effects significantly enhance the signal-to-noise ratio of the chip and minimize non-specific binding and diffusion artifacts.
A study by Steinicke et al.8 compared the long-term stability of pre-immobilized sensor chips, as well as their mechanical stability under repeated docking in a Biacore™ X100 instrument. XanTec sensor chips “offer a higher immobilization level although using the same immobilization assay.” “Furthermore, they show particularly constant values without any major variations,” leading to the conclusion that “the Biacore™ and the XanTec chips are supposed to behave equally.” In a further study9, it was stated that “that chips from Biacore™ (Cytiva®) as well as the chips obtained from XanTec are usable particularly for performance qualification” of SPR instruments.
However, for many applications XanTec’s sensor chips offer significantly added value. This is impressively demonstrated, for example, by the multidentate Ni-NTA sensor chips. Based on a strictly linear, flexible and hydrophilic polycarboxylate, His6-tagged proteins are immobilized with stability greater by 2–3 orders of magnitude than they are to carboxymethyldextran-based sensor chips as offered by Biacore™, which tend to so-called “leaching,” and are therefore not suitable for many applications. “The improved chemistry of the XanTec chips largely overcomes these limitations and allows the capture method to be employed for small molecule screening.”7 (Fig. 6).
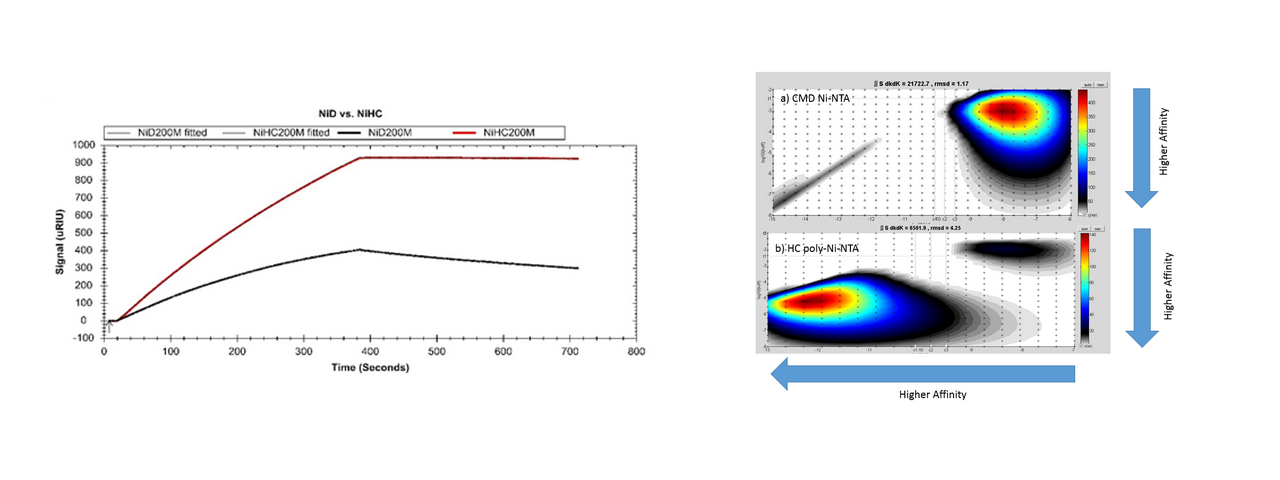
Not only is the underlying linear polycarboxylate (HC) a superior immobilization matrix for high-affinity Ni-NTA sensor chips, it is also a versatile and often better alternative to carboxymethyldextran. Because of the small molecular footprint, the extremely hydrophilic backbone, and the absence of other functional groups, diffusion characteristics and the suppression of non-specific interactions are significantly improved. In fact, the HLC variant with its decreased negative charge compared with the HC polymer is the surface with the lowest nonspecific interactions available today (test matrix: undiluted serum). As HC and HLC chips are not based on polycarbohydrates, carbohydrates or sugar-binding molecules such as lectins can also be studied, which is very difficult (often impossible) with dextran-coated sensor chips because of cross-reactivity effects.
| Compatible sensor chips and prisms |
|---|
| Biacore™ |
| BioNavis™ |
| Bruker™ / Sierra Sensors™ |
| Sartorius™ / ForteBio™ / SensiQ™ |
| Reichert SPR |
| BioRad™ ProteOn® |
| IBIS MX96® prisms |
| IBIS/Kinetic Evaluation Instruments™ |
| Horiba™ |
In addition to proprietary surface coating technology with the advantages described above, XanTec continues to develop solutions to surface-related problems and improved immobilization strategies. As just two examples, we mention our new, innovative zwitterionic surfaces and surfaces for click chemistry.
Pros of XanTec SPR sensor chips
- Better performance, i.e., cleaner curve fits and higher signal-to-noise ratios in many applications.
- Cross-platform availability – comparison of SPR data and protocols generated with different instrument platforms made easy.
- A sophisticated and significantly greater selection of surfaces allows better adaptation of the sensor chip to the planned experiment.
- Proven comparability and compatibility with Biacore™ sensor chips.
- Continuous improvement of existing products and innovative concepts for new chip chemistries.
- Significantly lower prices compared with the original manufacturer.
- Fast, worldwide shipping, usually within 3 business days.
- Complete solutions from a single source for your SPR experiments including buffers, reagents, and immobilization & regeneration kits.
Literature
- Brown, Michael E., et al. "Assessing the binding properties of the anti-PD-1 antibody landscape using label-free biosensors." PloS one 15.3 (2020): e0229206.
- Horáčková, V., Hlaváček, A., Čunderlová, V., Pastucha, M., & Skládal, P. (2017). Atomic force spectroscopic and SPR kinetic analysis of long circular and short ssDNA molecules interacting with single-stranded DNA-binding protein. Monatshefte Für Chemie - Chemical Monthly, 148(11), 2011–2018.
- Fischer, M. J. E. (2010). Amine Coupling Through EDC/NHS: A Practical Approach. Surface Plasmon Resonance, 55–73.
- Palau, W., Masante, C., Ventura, M., & Di Primo, C. (2013). Direct evidence for RNA–RNA interactions at the 3′ end of the Hepatitis C virus genome using surface plasmon resonance. RNA, 19(7), 982-991.
- Rusnati, M., Borsotti, P., Moroni, E., Foglieni, C., Chiodelli, P., Carminati, L., ... & Taraboletti, G. (2019). The calcium-binding type III repeats domain of thrombospondin-2 binds to fibroblast growth factor 2 (FGF2). Angiogenesis, 22(1), 133-144
- Test report “XanTec sensor chips” - tested in the Biacore Center of the GFB Biopharmaceuticals - 2008
- Doughty, L. (2020). Discovering inhibitors of cell surface receptor function as the basis for novel therapeutics to treat cancer (Doctoral dissertation).
- Steinicke, F., Oltmann-Norden, I., & Wätzig, H. (2017). Long term kinetic measurements revealing precision and general performance of surface plasmon resonance biosensors. Analytical biochemistry, 530, 94-103.
- Pögel, F. P., Oltmann-Norden, I., & Wätzig, H. (2018). Performance qualification for reproducible Surface Plasmon Resonance analysis. Analytical biochemistry, 544, 108-113.
Contact us today to make the most out of your assay and optimize your data. Our application specialists are available to answer any technical questions and will happily discuss your requirements.
Cytiva® is s trademark of Life Sciences IP Holdings Corporation or an affiliate
Biacore™ is a trademark of Global Life Sciences Solutions USA LLC or an affiliate