Introduction
OOne of the critical bottlenecks when developing chip surfaces for biosensor applications is the suppression of non-specific binding (NSB). A high background can dominate weak interactions, alter interaction profiles, and result in false positive signals. Suppression of NSB requires the use of well-defined surface chemistries, which, at the same time, provide sufficient surface capacity for ligand immobilization [1].
The commonest approach to decreasing non-specific adsorption while providing sufficient surface capacity for ligand immobilization is coating the surface with a hydrophilic polymer. Carboxymethyl dextran (CMD) hydrogels are a popular example [2]. A more recent generation of polyanionic hydrogels – the linear polycarboxylates (HC) – significantly improved the biocompatibility and diffusion characteristics of affinity biosensors.
However, even the most bioinert, usually polyanionic surfaces do not allow the direct covalent immobilization of very acidic species by, for example EDC/NHS1 immobilization chemistry. This severely limits the application scope of today’s coupling chemistries, because negatively-charged biomolecules, such as nucleic acids, proteins with low pI, and acidic polysaccharides, are of considerable interest.
1 EDC [1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide] in conjunction with NHS (N-hydroxysuccinimide) allows covalent coupling/immobilization of a primary amine-containing ligand to a carboxyl-functionalized surface.
The problem
To achieve a suitable immobilization efficiency and therefore high immobilization levels, electrostatically driven “pre-concentration” – an interaction between the negatively-charged sensor chip surface and (temporarily) positively-charged ligand – is essential. But acidic ligands (pI < 4.0) remain negatively charged in common immobilization buffers suitable for covalent EDC/NHS coupling, so it is extremely difficult (often impossible) to immobilize them covalently. This is a widespread problem that applies to thousands of acidic proteins [3]. Moreover, many more proteins with higher pI do not tolerate the acidic immobilization buffers normally used for EDC/NHS coupling.
The solution: a temporarily positively-charged surface
If the ligand remains negatively charged, then the surface needs to carry a net positive charge during the immobilization process to ensure sufficient electrostatic interaction to achieve a good immobilization yield. Afterwards, the surface should be neutral or slightly negatively-charged, as cationic surfaces are prone to nonspecific interactions.
With this in mind, XanTec has developed the zwitterionic ZC surface: a surface bearing positively-charged amine and negatively-charged carboxyl groups, i.e., an overall neutral net charge (Fig. 1a, left). Zwitterionic, neutral surfaces are well known for their improved bioinertness compared with, for example, negatively-charged CMD surfaces [4].
The trick: when activated for covalent immobilization, the negatively-charged carboxylic groups on the surface are transformed into uncharged, reactive NHS esters – resulting in a positive charge surplus (Fig. 1a). Now, a “reversed charge” pre-concentration of negatively-charged ligand (such as protein or nucleic acid) is enabled, drastically increasing the local ligand density [5], which results in high immobilization yield.
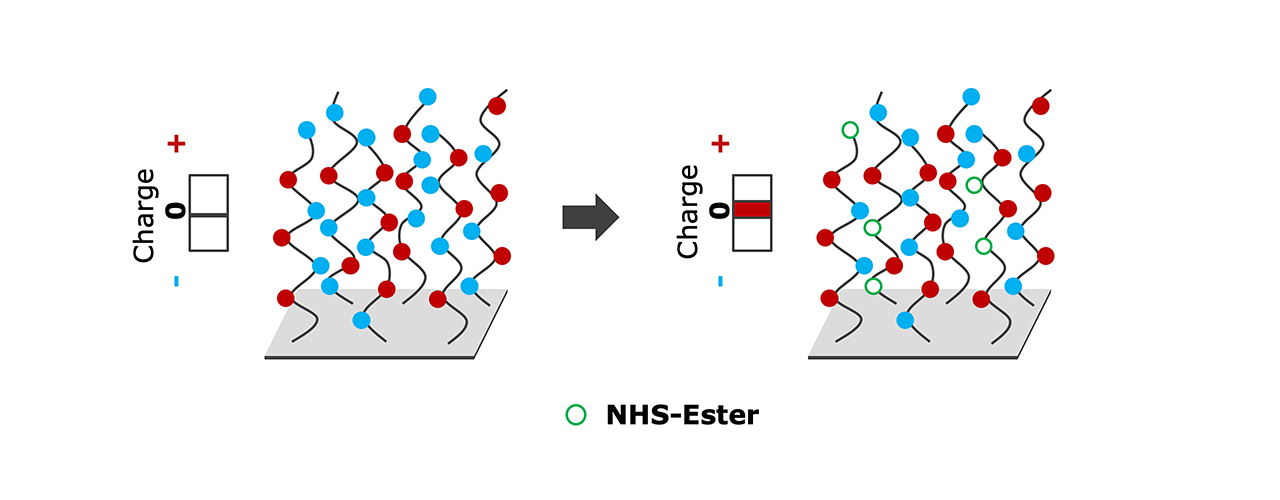
- Activation of the initially neutral surface for immobilization, and conversion to net positive charge.
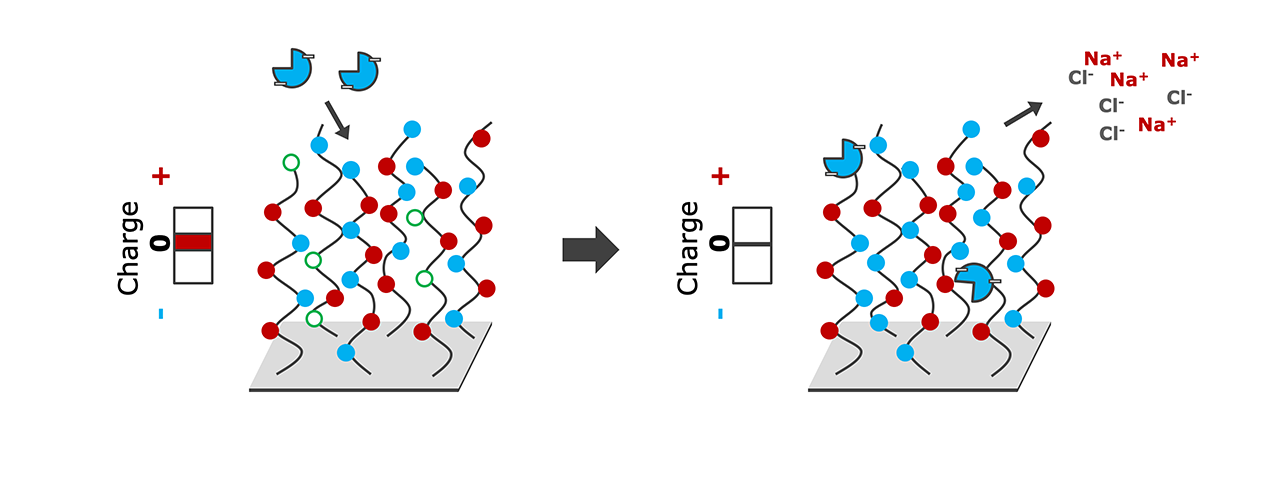
- Pre-concentration and covalent immobilization of negatively-charged ligands on the now positively-charged surface. Right: Neutral surface with immobilized ligand after deactivation/hydrolysis of remaining reactive esters.
A major advantage of “reversed charge” immobilization is that it is possible to immobilize almost all acidic ligands at physiological pH values, which is not only more gentle, but also contributes positively to the immobilization efficiency, compared with standard EDC/NHS immobilization conditions. To achieve a sufficient “reversed charge” for pre-concentration on zwitterionic ZC sensor chips, the pH of the immobilization buffer should be 1–1.5 pH units above the pI of the ligand.
For deactivation of unreacted esters and to bring the surface charge back to neutrality, the remaining NHS esters are hydrolyzed in moderately alkaline buffer (pH 8.0) for a few minutes, or at physiological pH for 1 h (Fig. 1b).
Bioinertness of zwitterionic sensor chip surfaces
The positive impact of such zwitterionic surfaces in decreasing NSB – especially of positively charged proteins – is shown in Fig. 2. Three different proteins were incubated for 10 min on the zwitterionic, neutrally-charged ZC surface with no NSB (left). For comparison, lysozyme was incubated for 10 min and, on a negatively-charged CMD surface, which showed a distinct non-specific interaction.
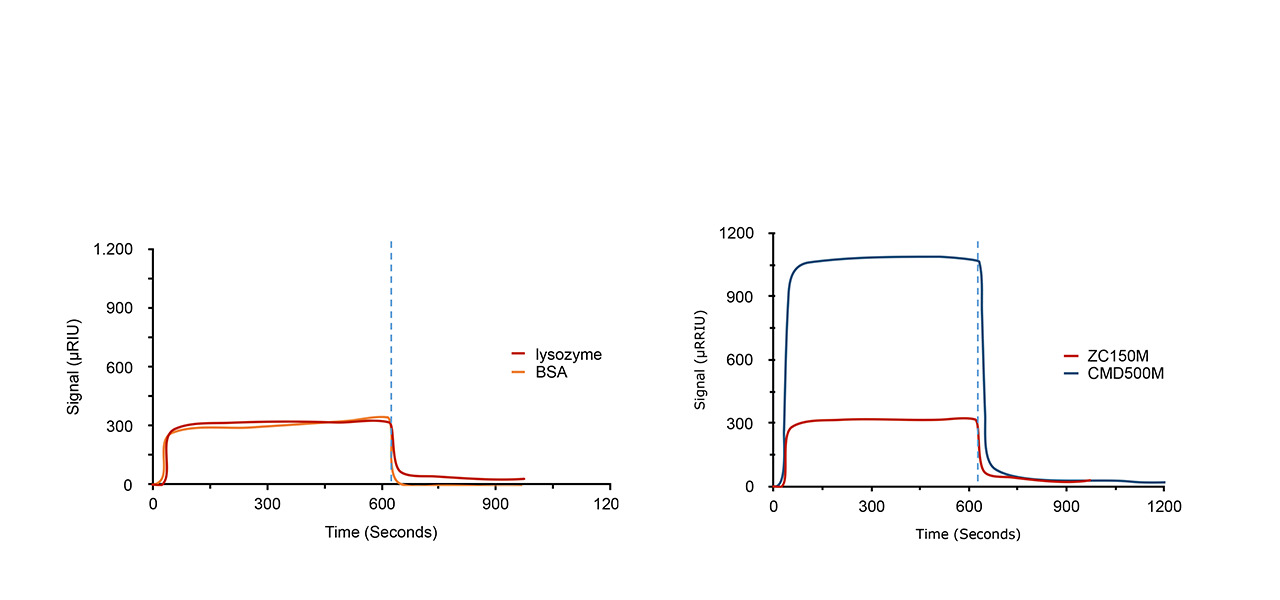
2 HBS: HEPES buffered saline
Applications
Pepsin is an acidic protein that has not previously been attached to carboxyl surfaces by conventional covalent EDC/NHS immobilization [5]. The low pI of this endopeptidase (2.0–2.5) makes it impossible to create a net positive charge on the protein and at the same time a negative charge on a carboxylate sensor chip for pre-concentration-mediated efficient coupling. By using “reversed charge” pre-concentration on the zwitterionic ZC surface, this protein was effectively immobilized for the first time via EDC/NHS chemistry (Fig. 3).
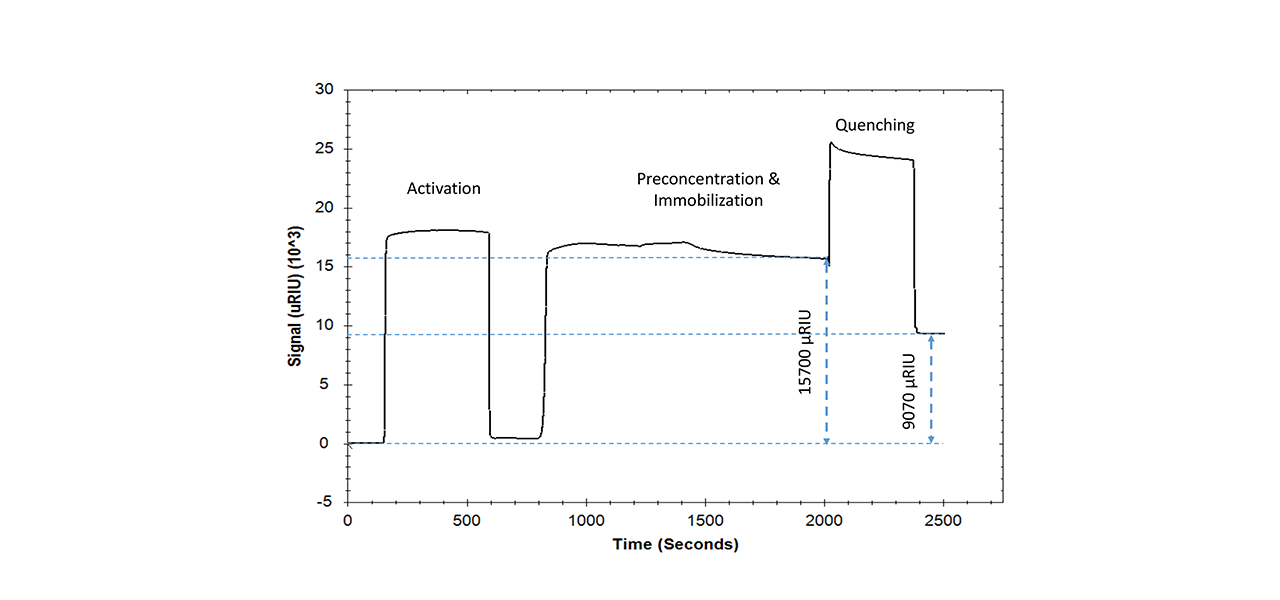
Nucleic acids (including related aptamers) are another class of biomolecule that is usually not accessible using pre-concentration-enhanced covalent coupling, because the high negative charge density of their phosphate backbones does not allow for electrostatic pre-concentration on negatively-charged surfaces. Fig. 4 shows the immobilization of an amino-modified 16mer ssDNA oligo on the ZC surface where 3090 µRIU (RU) were preconcentrated and 1220 µRIU (RU) covalently immobilized.
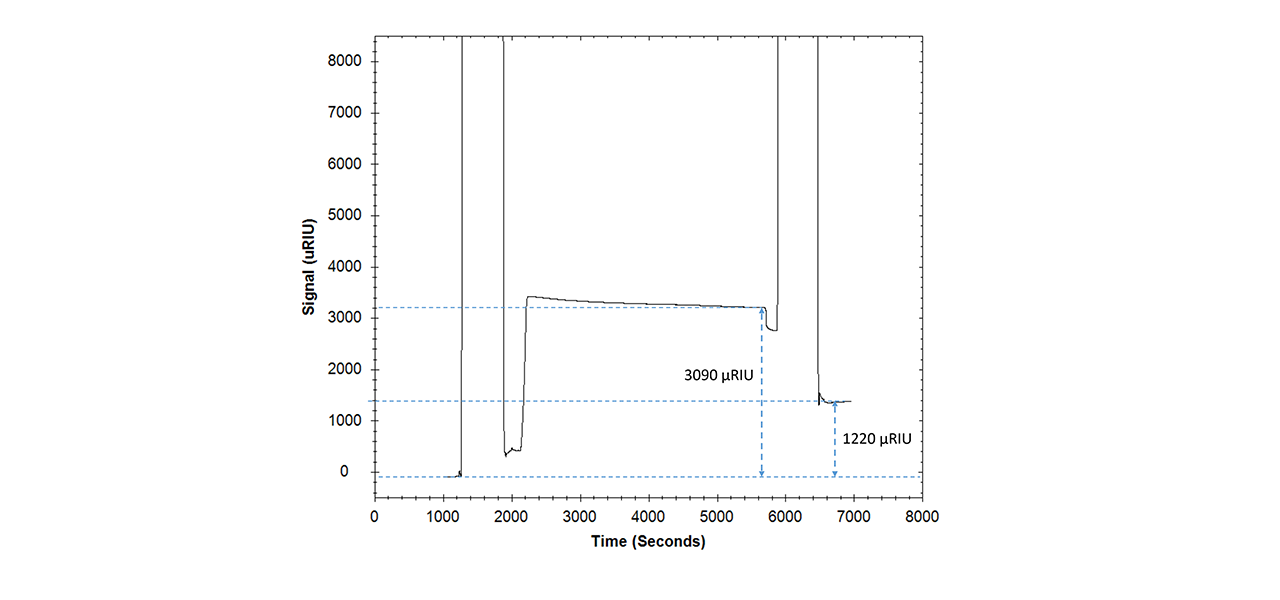
In a subsequent hybridization experiment with the complementary DNA sequence (anti-ssDNA), the binding characteristics of the ssDNA immobilized on the zwitterionic ZC surface (Δ1220 µRIU) were compared with a CMD-based standard streptavidin sensor chip derivatized with an equivalent amount of biotinylated ssDNA (Δ1210 µRIU). The resulting hybridization/binding isotherms are shown in Fig. 5.
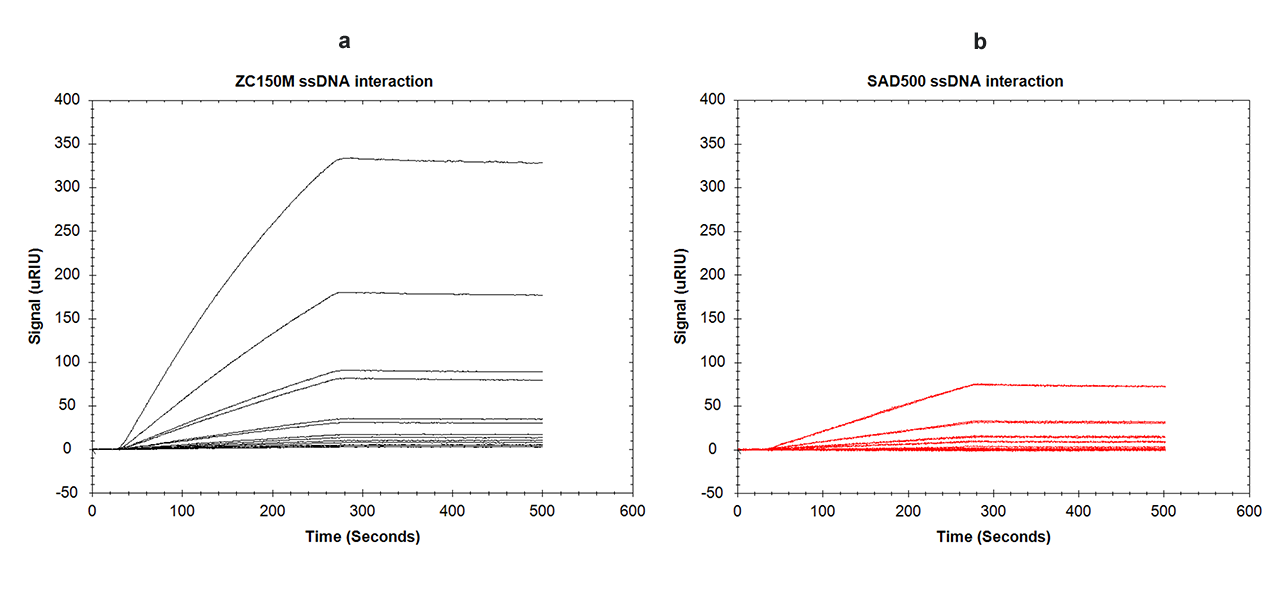
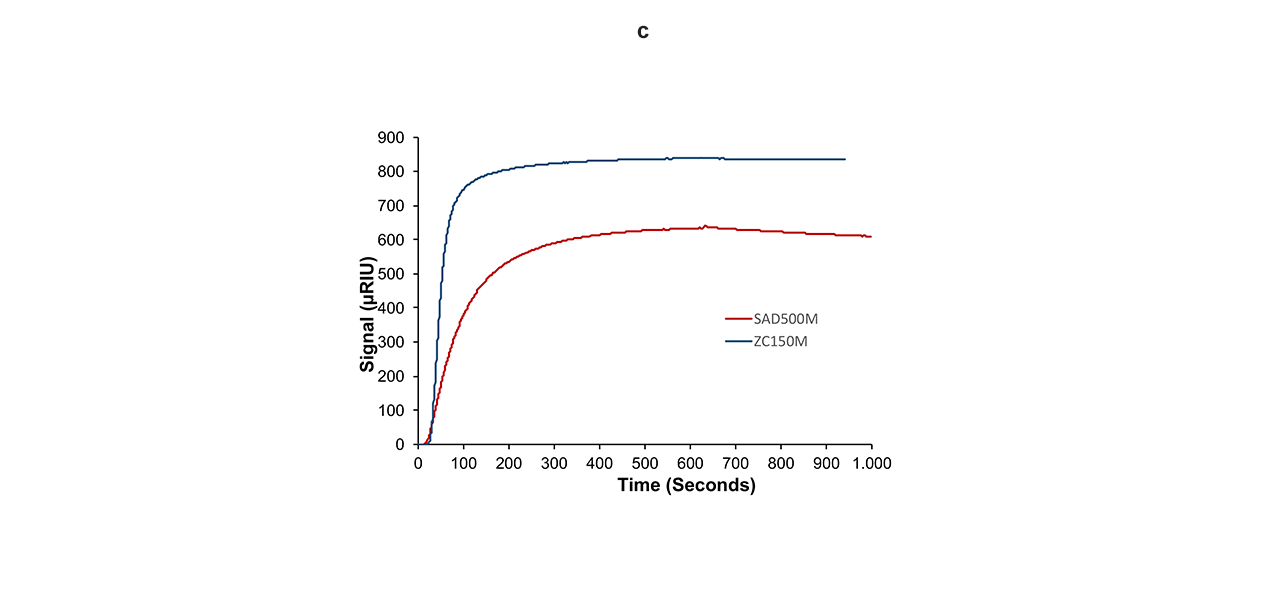
Figure 5c. Comparison of the hybridization of 1 µM anti-ssDNA with its complementary ssDNA using “reversed-charge” immobilization (blue line) and a streptavidin-modified CMD sensor chip (red line).
The difference between the two interactions becomes apparent at first glance. The total amount of bound anti-ssDNA was 35% higher on the ZC sensor chip than on the streptavidin chip (Fig. 5c). This can only be explained by a higher activity of the ligand [5]. In addition, the shapes of the binding isotherms on the ZC sensor chip are also significantly steeper compared with the streptavidin approach (Fig. 5a–c). This indicates a higher association rate, which implies better accessibility/activity of the ligand. Compared with the CMD-based streptavidin surface, the ZC hydrogel has a more open structure, resulting in significantly less steric hindrance, better diffusion, and a higher apparent association rate.
Conclusions
The above applications show that zwitterionic ZC surfaces solve several major problems associated with standard EDC/NHS immobilization chemistry:
- Electrostatic pre-concentration and direct immobilization of negatively-charged (acidic) ligands becomes possible by simply reversing the charge of the surface upon EDC/NHS activation.
- The fact that ligands no longer need to be positively charged for covalent immobilization also opens up new immobilization options for highly pH-sensitive proteins. Many of them can now be immobilized at physiological pH.
- Because of efficient suppression of electrostatic interactions compared with purely polyanionic surfaces, zwitterionic hydrogels better suppress non-specific binding of positively-charged analytes or sample components.
With zwitterionic ZC sensor chip surfaces, XanTec has opened the possibility of covalent immobilization of acidic and/or pH-sensitive ligands. This ability will significantly broaden the experimental options in biomolecular interaction analysis.
Zwitterionic sensor chips are available as ZC30M, ZC80M and ZC150M.
References
- Joshi: Surface characterization and antifouling properties of nanostructured gold chips for imaging surface plasmon resonance biosensing, Sensors and Actuators B: Chemical (2015) 209, 505–514.
- Löfås, Johnsson: A novel hydrogel matrix on gold surfaces in surface plasmon resonance sensors for fast and efficient covalent immobilization of ligands, J. Chem. Soc. Chem. Commun. (1990) 1526–1528.
- http://isoelectricpointdb.org/40/UP000005640_9606_all_isoelectric_point_proteome_Homo_sapiens_Human.html
- Schlenoff: Zwitteration: coating surfaces with zwitterionic functionality to reduce nonspecific adsorption, Langmuir (2014) 30(32), 9625–9636.
- Risse, Gedig, Gutmann: Carbodiimide-Mediated Immobilization of Acidic Biomolecules on Reversed-Charge Zwitterionic Sensor Chip Surfaces, Anal Bioanal. Chem. (2018) 410(17), 4109–4122.