Miniaturized, referenced, SPR based self interaction chromatography (SPR-SIC)

The use of proteins in industrial and pharmaceutical applications has become increasingly common. For example, a multitude of monoclonal antibodies (mAbs) are currently in biopharmaceutical drug development due to their high specificity and binding strengths. However, antibodies tend to aggregae and degrade at elevated concentration, and, as a consequence, lose their potency. In addition, protein drug formulations containing aggregates are known to cause an immunogenetic response and cannot be used. Preparation of very high concentrations of antibodies at a very low volume per dose is critical.
Therefore, predicting or quickly measuring aggregation/self-interaction behavior is preferred to have suitable formulations in the development of protein-based biopharmaceuticals [1].
Experimental methods for characterizing the influence of solution conditions (pH, ionic strength, etc.) would provide researchers with the opportunity to make predictions about how these variables will influence solubility, phase behavior and eventual crystallization. Protein-protein interaction phenomena occur throughout the full range of pharmaceutical protein-processing environments [2].
Protein-protein self-interactions under varying conditions are important, and as a screening tool to analyze these interactions self interaction chromatography (SIC) has been established for many years. But this method has the great disadvantage that very large amounts of protein are needed and the possibility for referencing is limited. With the fully automated surface plasmon resonance (SPR) based self interaction chromatography (SPR-SIC) these boundaries could be overcome.
SPR-SIC method
SPR, also known as the "Biacore" method, is widely associated with the determination of kinetics and active concentrations of proteins, peptides, and small drugs. Usually, these instruments are less flexible, closed black boxes that allow no other use or flexible design of experiments.
Not so, with the new and highly flexible SPR-PLUS concept, which was developed in collaboration with several leading pharmaceutical companies.
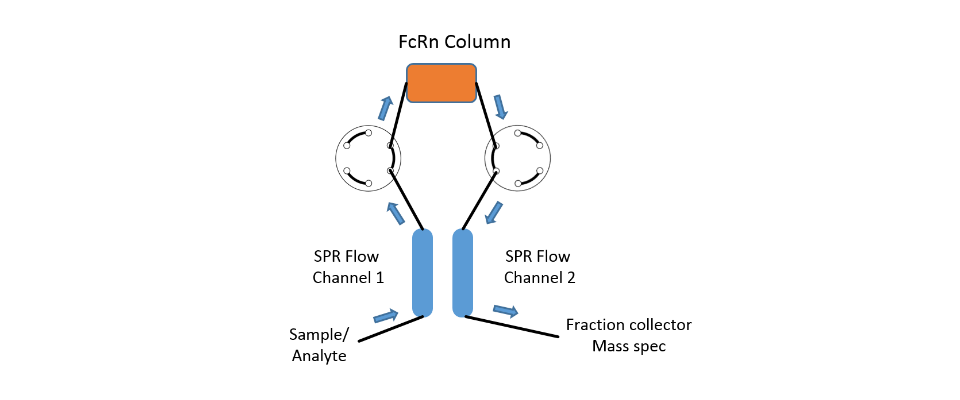
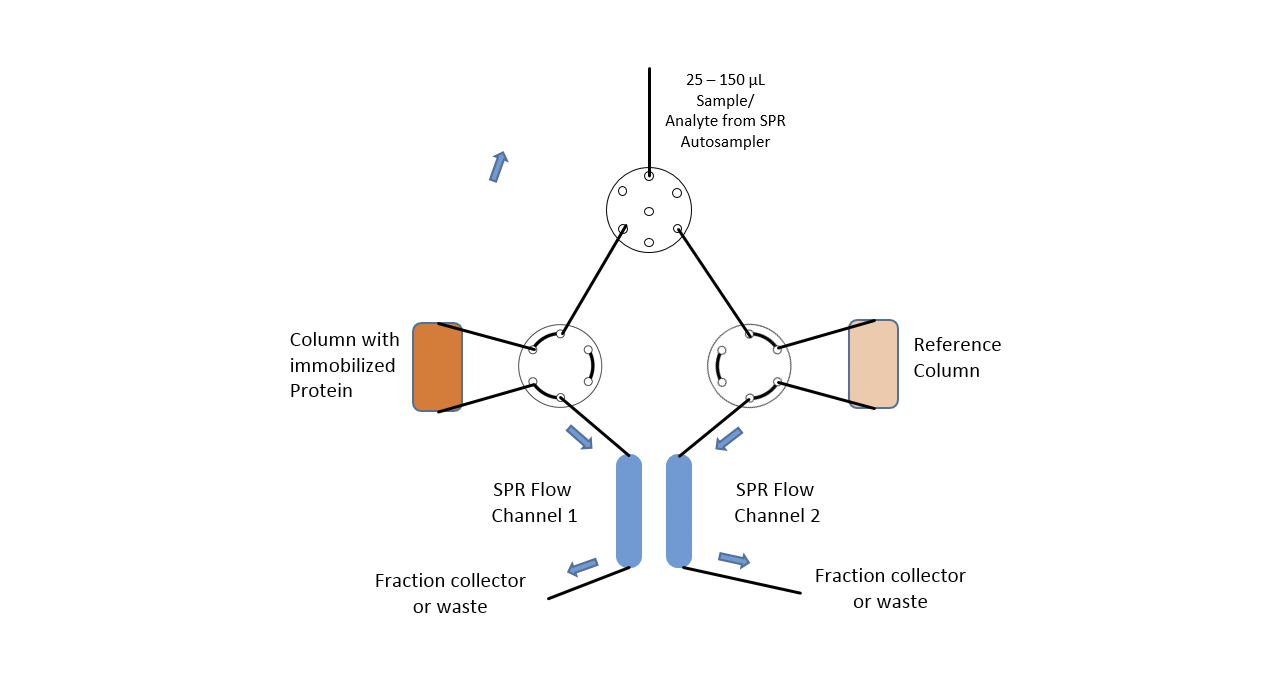
In this case, in addition to the high sensitivity of the SPR system, the extreme flexibility of the fluidics of the SPR-PLUS concept is exploited. Here, two μ-columns are integrated between the flow channels of the SPR system by means of an extended valve module, which serve as a reference and interaction column. Wherein the signal on the first SPR channel serves as a control for possible variations of the backpressure / packing density of the two columns and the second column downstream of the columns registers the retention time differences between the two columns.
The immobilization of the protein takes place automatically for the SPR flow channels through the autosampler. In the protein-loaded column, it is possible to choose between online immobilization and manual offline immobilization. If a calibration of the two columns is necessary this is done with injections of 1 M NaCl and 5% glycerol. The immobilization chemistry is dependent on the surface chemistries of both SPR sensor chip and used column material.
Materials and Methods
The system was equipped with two μ-column holders as shown in Figure 1. The μ-columns were filled with in each case 75 μL of CM-Sephadex 50 suspended in PBS. The column material for both columns was activated with 2% EDC in NHS / MES buffer (XanTec). For the protein column 100 μL of column material was washed several times with 5 mM sodium acetate pH 5.0 and then incubated with 200 μL of a 100 µg/mL mAb1 solution for 15 minutes. Subsequently, both column materials were quenched with 300 μL 1M ethanolamine, pH 8.5 to eliminate residual reactive groups.
Finally, the treated column materials were washed several times with PBS and then introduced into the columns. The antibody was immobilized online using a similar protocol on the sensor chip.
After incorporation of the column cartridges into the respective column holders, the entire SPR system was rinsed with 10 mM histidine as an air buffer. After equilibration of the columns and chip surface, different concentrations of the antibody (400, 200, 100, 50, 25, 12.5 μg / mL) were added for 4 min at 20 μL/min each over the protein and reference columns. Any non-specifically bound antibody was eluted by injections of 500 mM NaCl, 0.1 M Na borate, pH 7.8.
Results
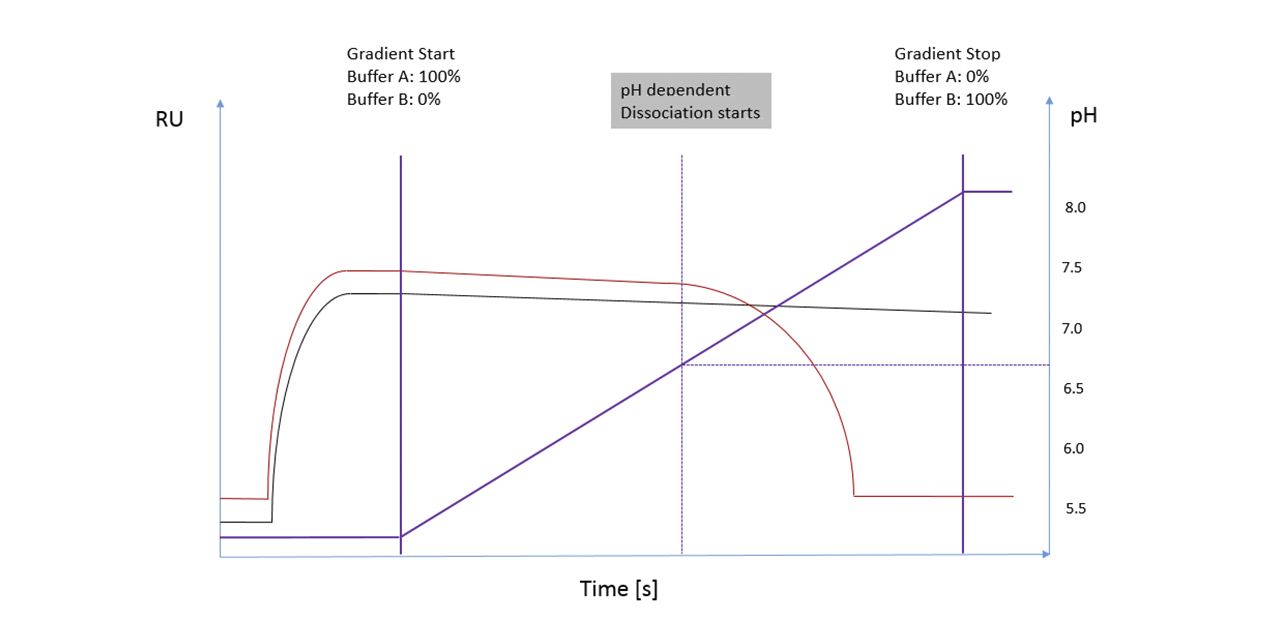
The overlayed SPR sensorgrams (Fig. 2) show concentration dependent signals (peaks on the left side / refractive index jumps by concentration and non-specific binding) on the first SPR flow channel before the protein is in contact with the either the mAb-modified or reference column. The reproducibility of the injections for both flow paths (CH1-mAb-column-CH2 / CH1-reference-Column-CH2) indicates that both columns have the same backpressure. The peaks on the right side of the sensorgram (combination of refractive index by concentration and non-specific binding) show clearly different retention times between the reference and the mAb-modified column.
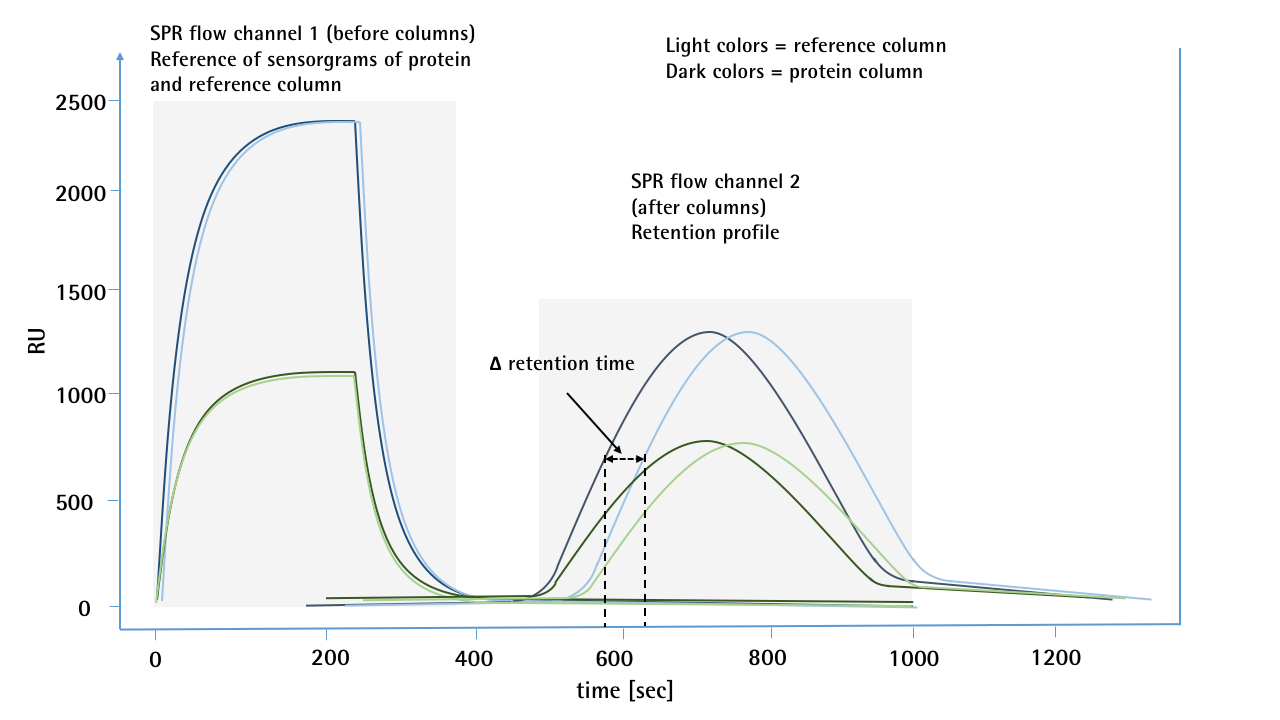
Figure 2: SPR sensorgrams of 2 different concentrations (10 & 50 µg/mL) of a mAb for self-interaction chromatography. For each concentration, a sample was first passed through the 1st SPR flow channel, then passed over a protein or reference column and then transferred to the 2nd flow channel. By means of salt injections, the different flow paths between the reference and the protein columns were previously calibrated and no deviation in the retention time was observed. The first flow channel of the SPR serves as a pre-column detector and shows no deviation for the two injections of one concentration, which would indicate any backpressure differences in the flow paths. The sensorgrams of the 2nd flow channel show a clear deviation in the retention time. In this case, the sample which was passed over the protein column was much more likely to reach the second flow channel than the sample which was passed over the unmodified reference column.
Summary and Conclusion
Understanding weak protein interactions is important for treating biological, crystallizing proteins, including for structure-based drug design, purifying protein mixtures, understanding protein diffusion in concentrated solutions, and stabilizing protein therapeutic formulations. However, these interactions are typically too weak to characterize in terms of quantities such as association constants that are suitable for strong protein interactions measured using methods such as surface plasmon resonance (SPR) [4]. Instead, of using SPR to characterize these weak protein interactions in the classic way, the SPR spectrometer was utilized as an ultra-sensitive detector for a miniaturized version of classical self-interaction chromatography (SIC) to characterize the interactions in terms of the osmotic second virial coefficient (B22). The new SPR concept significantly reduces the required amount of sample material and can therefore be located even in an earlier stage than in formulation development and also shows the application possibilities for other chromatographic questions.
- Deshpande, K. S., Ahamed, T., Ter Horst, J. H., Jansens, P. J., Van Der Wielen, L. A., & Ottens, M. (2009). The use of self‐interaction chromatography in stable formulation and crystallization of proteins. Biotechnology journal, 4(9), 1266-1277.
- Cleland, J. L., Powell, M. F., & Shire, S. J. (1993). The development of stable protein formulations: a close look at protein aggregation, deamidation, and oxidation. Critical reviews in therapeutic drug carrier systems, 10(4), 307-377.
- Ahamed, T., Ottens, M., van Dedem, G. W., & van der Wielen, L. A. (2005). Design of self-interaction chromatography as an analytical tool for predicting protein phase behavior. Journal of Chromatography A, 1089(1-2), 111-124.
- Tessier, P. M., Lenhoff, A. M., & Sandler, S. I. (2002). Rapid measurement of protein osmotic second virial coefficients by self-interaction chromatography. Biophysical journal, 82(3), 1620-1631.