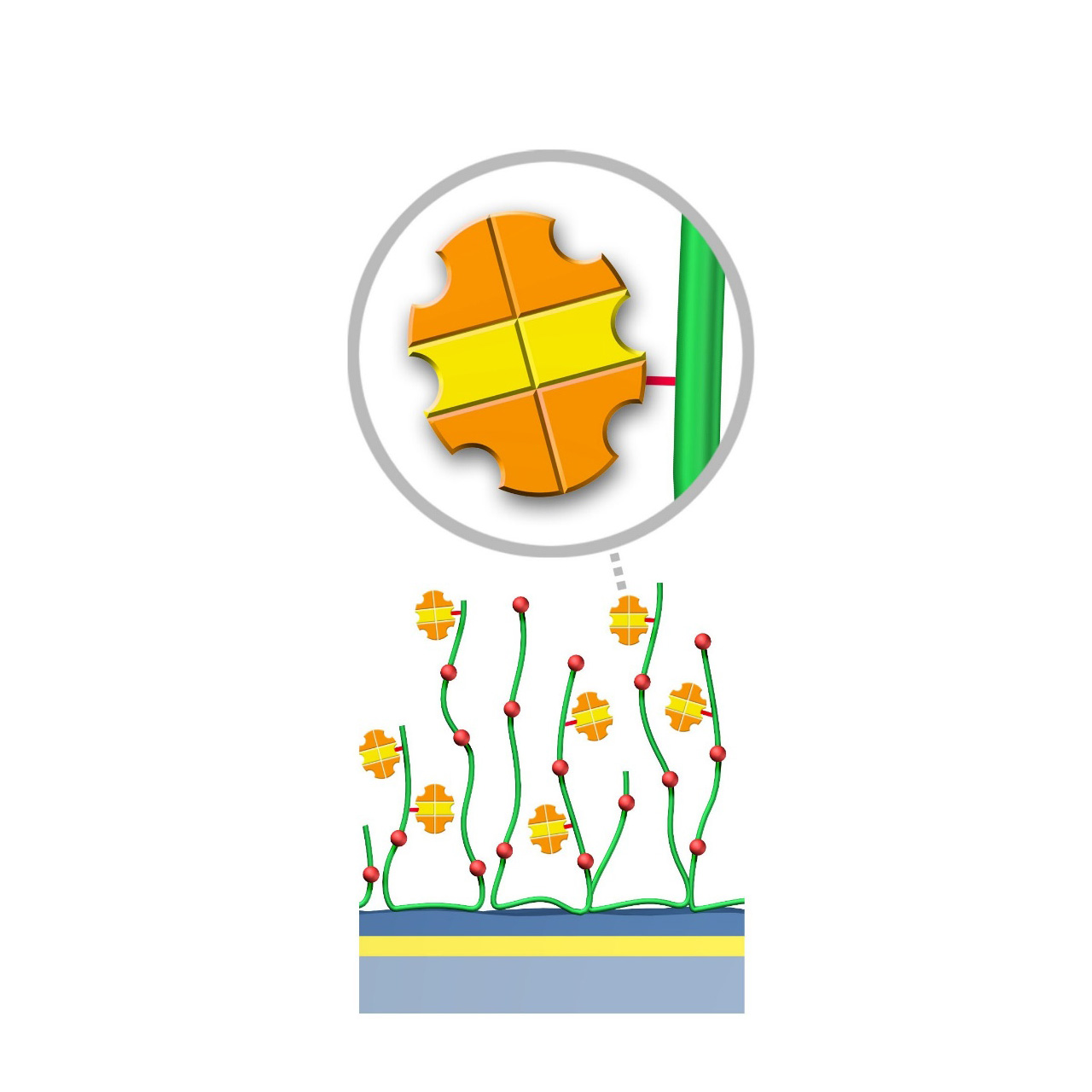
Protein AG–modified sensor chips
XanTec’s Protein AG (PAG) sensor chips are coated with a bioinert polycarboxylate matrix pre-functionalized with a recombinant 50.5 kDa Protein AG. This non-glycosylated variant combines four IgG-binding domains from Protein A and two IgG-binding domains from Protein G, providing high-affinity binding to the Fc region of all human IgG subclasses. In addition, it exhibits strong affinity for mouse IgG2a, IgG2b, and IgG3, as well as total IgG from cow, goat, sheep, rabbit, and other mammals.
To ensure highly specific IgG binding, non-essential domains (e.g., cell wall-, cell membrane-, and albumin-binding regions) have been removed from the recombinant Protein AG. Nevertheless, due to its slightly hydrophobic character, some nonspecific binding may occur. The inclusion of blocking agents (e.g., BSA) and/or nonionic detergents (e.g., Tween) in the running buffer is therefore recommended to minimize background signals. XanTec’s PAG sensor chips are ready-to-use, enabling rapid assay setup without time-intensive surface optimization.
Key features:
- Fast assay development: No preconcentration or surface activation required; controlled and reversible capture under physiological conditions.
- Broad antibody compatibility: Wider subclass and species coverage than Protein A.
- Simple regeneration: Regeneration using glycine·HCl pH 1.5.
- High stability: Robust capture surfaces support repeated regeneration cycles.
- Oriented immobilization: Directed Fc-region binding ensures high ligand activity.
| Product code | PAGP | PAGD200L | PAGHC30M | PAGHC200M |
|---|---|---|---|---|
| Base coating | 2D, ultra-short bioinert CM-dextran (high density) |
3D, 200 nm bioinert CM-dextran (low density) |
3D, 30 nm bioinert polycarboxylate (medium density) |
3D, 200 nm bioinert polycarboxylate (medium density) |
| Capture immobilization capacity [µRIU]3 | ≈ 5,000 | ≈ 12,000 | ≈ 6,000 | ≈ 11,000 |
| Ligands | Fc region of all human IgG subclasses; strong affinity for mouse IgG2a, IgG2b, IgG3, and total IgG from cow, goat, sheep, and rabbit | |||
| Recommended analytes |
|
|
|
|
| Intended purpose |
|
|
|
|
1 All illustrations are schematic representations and are not drawn to scale; dimensions, densities, and spatial relationships do not reflect actual physical or chemical proportions.
2 Table includes a selection from XanTec’s full PAG sensor chip portfolio.
3 Maximum immobilization capacities are based on capture from a 100 µg/mL solution of Protein A-purified, pooled rabbit IgG in PBS, with 1 µRIU corresponding approximately to 1 RU.