Protein tags have been an important component in the design of recombinant fusion proteins for decades. In addition to simplified detection (e.g., using GFP tags), short peptide tags facilitate purification, downstream quantification, and immobilization of the fusion protein. In the context of SPR spectroscopy, divalent nickel ion-mediated immobilization of His6-tagged proteins on NTA-functionalized sensor coatings is well-known. In contrast to the classical, covalent EDC/NHS coupling, immobilization via the His-tag occurs in physiological conditions, is site-specific, and reversible. However, these advantages are counteracted by the relatively low stability of the His6–Ni2+ complex (kd ≈ 10−3 s−1) and an associated baseline drift, as well as (sometimes) high nonspecific interactions, especially with larger analytes. Although the development of polyNTA coatings (kd ≈ 10−6 s−1) has resulted in drastically increased binding stability, the system-inherent high nonspecific interactions with sterically available histidines of protein analytes remain problematic.
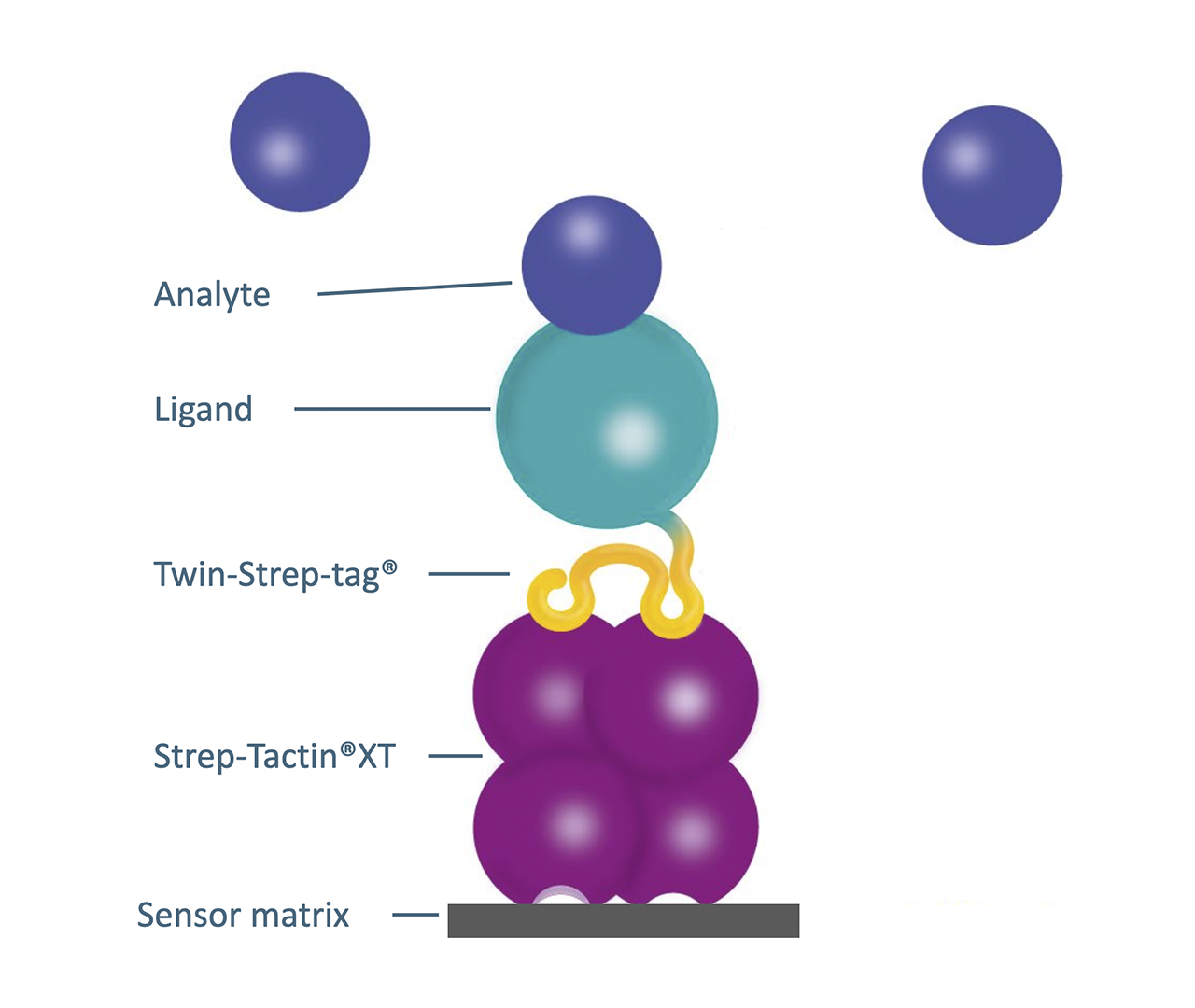
Aware of these issues, XanTec bioanalytics have teamed up with IBA Lifesciences to introduce their Strep-Tactin®XT (SXT)/Twin-Strep-tag® (TST) system as a new derivative on SPR sensor chips. The Strep-Tactin®XT/Twin-Strep-tag® system combines exceptionally high affinity (in the low picomolar range) and high specificity with efficient regenerability in mild conditions. This unique combination not only makes it attractive for affinity-based purification of recombinant Twin-Strep-tag®1-functionalized fusion proteins (typically >95% purity after the first run), but is also excellent for the often time-consuming and challenging protein immobilization on SPR sensor chips.
XanTec has extensively evaluated the SXT/TST system for SPR biosensor applications and made it commercially available to the SPR community in the form of three well-matched Strep-Tactin®XT sensor chip variants (STD200L, STHC200M, and STP).
We offer the Strep-Tactin®XT sensor chips as a carboxymethyl dextran-based version (STD200L), and as a derivative of a structurally different polycarboxylate hydrogel with very low nonspecific interactions (STHC200M). These STD/STHC sensor chips, which are optimized in terms of immobilization capacity and diffusion characteristics, are based on a three-dimensional polycarboxylate sensor matrix, and allow the reversible immobilization of typically several thousand µRIU of a TST fusion protein. They thus allow the user to address a wide range of biologically relevant systems and questions, such as protein–protein interactions, protein–peptide interactions, fragment screening (fragment-based lead discovery), and simple concentration analyses. Meanwhile, the two-dimensional low-capacity CMDP variant (STP) has particularly good diffusion characteristics for larger analytes.
1 Amino acid sequence of Twin-Strep-tag: WSHPQFEK-GGGSGGGSGG-SA-WSHPQFEK
Provided that one establishes the corresponding expression system (Twin-Strep-tag®), Strep-Tactin®XT sensor coatings impress with their unique property profile:
- Outstanding binding stability: kd ≈ 10−5–10−7 s−1
- Straightforward regeneration: e.g., 3 M Guanidine*HCl for 60 s
- High repeatability: Approx. 0.1% capacity loss per regeneration cycle; >100 regenerations possible
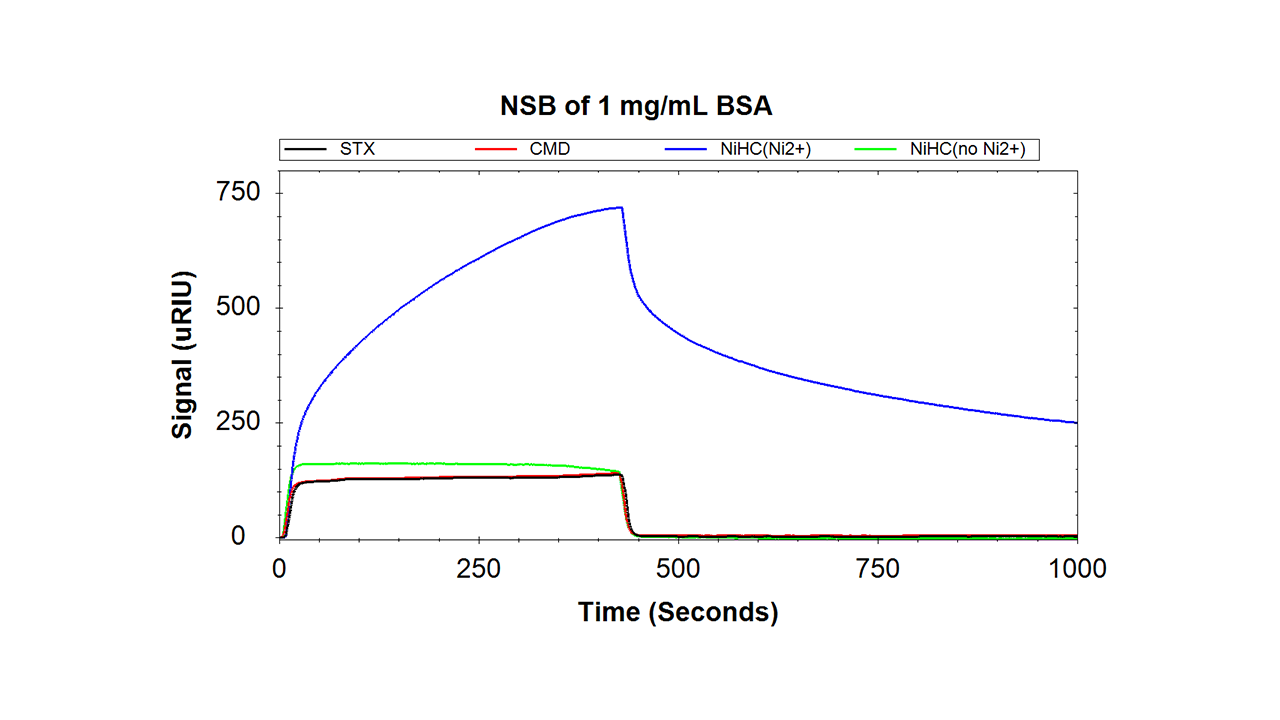
- Low nonspecific interactions2: no increase of nonspecific interactions compared with the underlying coating. Strongly decreased nonspecific interactions compared with NTA(Ni2+) derivatives
- Site-specific immobilization: No crosslinking; high biological activity, typically >70%
- Excellent control over the immobilization density
- Immobilization in physiological conditions: no preconcentration necessary
- Direct immobilization from complex media is possible
2 Based on investigations of nonspecific interactions of 50% fetal bovine serum (FBS), 1 mg/mL bovine serum albumin (BSA), and 1 mg/mL lysozyme in HBSEP buffer.
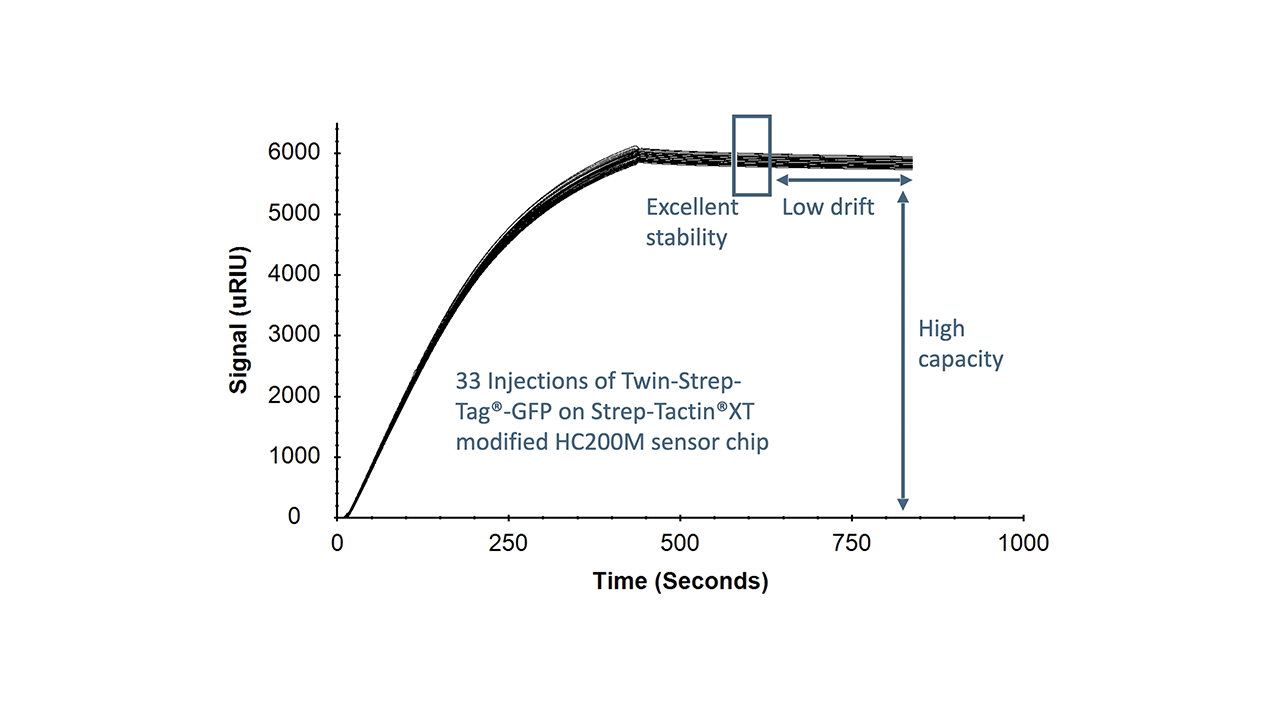
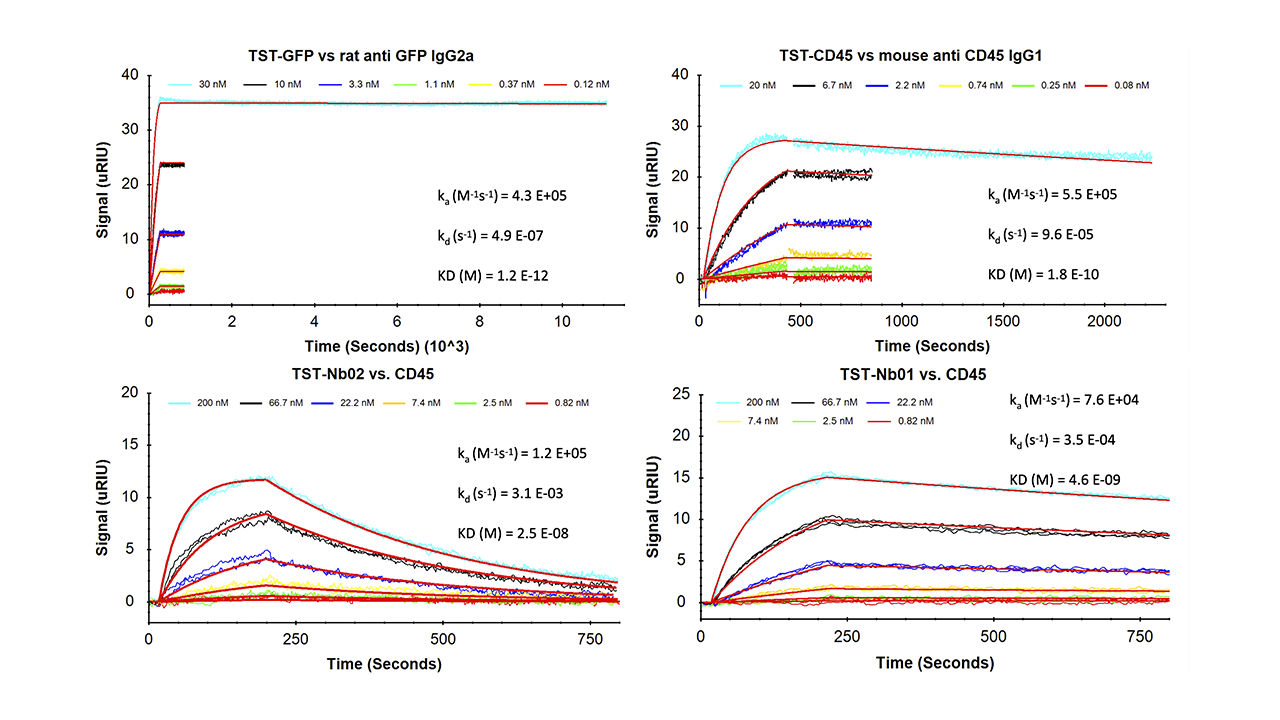
The SXT/TST system thus has all the positive aspects of His-tag/NTA(Ni2+) immobilization without its inherent disadvantages. This unique property profile makes it one of the most convenient and powerful SPR immobilization methods available. TST fusion proteins can be immobilized highly reproducibly in minimal time while preserving maximum biological activity. The overlay plots of four model kinetic interaction analyses shown in Fig. 4 illustrate the reliability of this immobilization method. The data generated all follow a 1:1 binding model. In particular, the interaction analysis between a TST-GFP protein and a rat anti-GFP IgG2a (Fig. 4, upper left) demonstrates the excellent stability of the Strep-Tactin®XT/TST system.
A wealth of further information on the entire recombinant protein production chain using the Strep-Tactin®XT/Twin-Strep-tag® system can be found at www.iba-lifesciences.com.
Selected literature
- Laffeber, C.; de Koning, K.; Kanaar, R.; Lebbink, J. H. G. Experimental Evidence for Enhanced Receptor Binding by Rapidly Spreading SARS-CoV-2 Variants. Journal of Molecular Biology 2021, 433 (15), 167058. https://doi.org/10.1016/j.jmb.2021.167058.
- Schmidt, T. G. M.; Eichinger, A.; Schneider, M.; Bonet, L.; Carl, U.; Karthaus, D.; Theobald, I.; Skerra, A. The Role of Changing Loop Conformations in Streptavidin Versions Engineered for High-Affinity Binding of the Strep-Tag II Peptide. Journal of Molecular Biology 2021, 433 (9), 166893. https://doi.org/10.1016/j.jmb.2021.166893.
- Yeliseev, A.; Zoubak, L.; Schmidt, T. G. M. Application of Strep-Tactin XT for Affinity Purification of Twin-Strep-tagged CB2, a G Protein-Coupled Cannabinoid Receptor. Protein Expression and Purification 2017, 131, 109–118. https://doi.org/10.1016/j.pep.2016.11.006.
Contact us today to make the most out of your assay and optimize your data. Our application specialists are available to answer any technical questions and will happily discuss your requirements.