SPR and biosensors
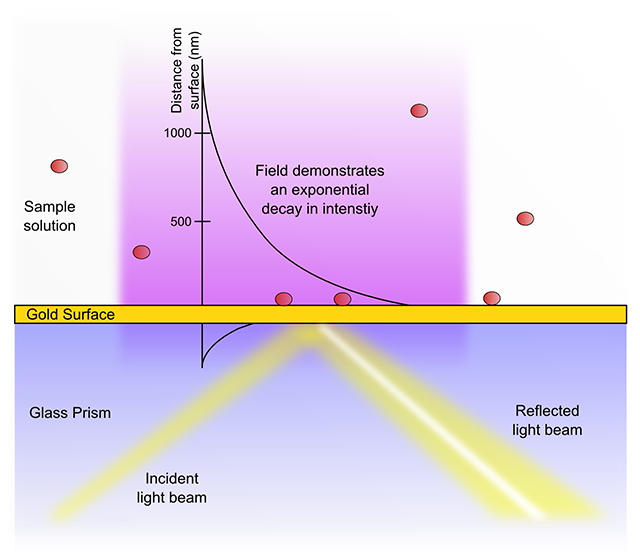
SPR biosensors work measure alterations in the SPR angle as a sample passes over and analyte molecules bind to immobilized ligands at or close to the sensor surface. One important consequence of the exponential decay of the evanescent field is that a typical SPR biosensor is unable to detect events beyond 600 nm from the sensor chip surface. The high signal-to-noise ratios achievable with evanescent field sensors are partially due to this relative insensitivity towards changes in the sample bulk. In addition, identical interactions at differing distances from the sensor surface will give rise to different signal shifts. Thus a receptor-ligand binding event (or indeed non-specific interactions) within 10 nm of the metal surface will result in an almost three times greater response than the same process at 300 nm distance.
Different coatings on the sensor chip allow adaptation of the sensor to different ligand/analyte systems, and so allow the ‘tuning’ of the detector. The potential ligands which can be coupled to various surfaces, and thus the detectable analytes, are effectively unlimited – and so SPR biosensors have been used to analyse binding of samples ranging from small proteins through to entire cells. As the method works in real time, successive sample and buffer injections can be used to calculate properties such as association and dissociation rate constants – and from these the affinity constant (Ka) can be determined.
The degree by which the SPR angle changes as a function of the change in refractive index is dependent on the light wavelength and the sensor chip itself. Thus comparison of results between different protocols requires calculation of the overall refractive index change (in µRIU) from the measured SPR angle shift (in millidegrees). Alternative units such as resonance units (RU), which are apparent and not related to SI units, are also in use.