Using Combined Electrochemistry and SPR to Monitor the Electropolymerization
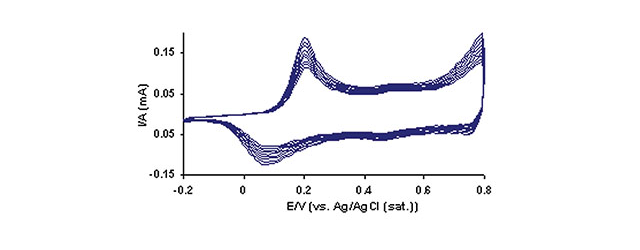
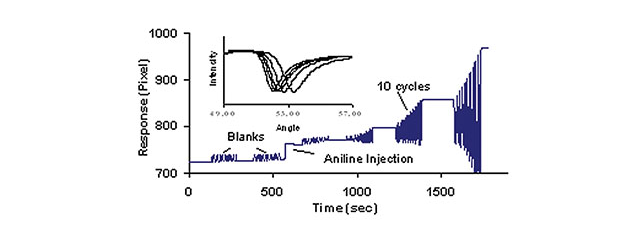
Conducting polymer thin films have been the focus of increased investigation because of their importance to a variety of applications including displays, batteries and sensors. Polyaniline (PAn) is a conducting polymer that has been widely studied because of its unique properties. These features include its relative ease of synthesis, remarkable environmental chemical stability, and the drastic change in its electrical conductivity upon simple oxidation and reduction. Owing to the SR7000DC’s open architecture, XanTec offers specialized flow cells/wells for performing other measurements in combination with surface plasmon resonance (SPR). This Application note presents the combination of electrochemistry and SPR to monitor the growth of a PAn film on the surface of bare gold with the SR7000DC system. Specifically, cyclic voltammetry (CV) is used as the deposition method while SPR simultaneously monitors the growth of the PAn film in real time.
Experimental
The CV/SPR experiments were carried out utilizing a three-electrode system with the gold surface as the working electrode, a platinum wire as the counter electrode and a Ag/AgCl reference electrode. The potential was cycled between -0.2 and 0.8 V 10 times and the SPR baseline was established for at least 4 min before the next set of potential cycles. 6 total sets of cycles were carried out including 2 blank cycle sets before the aniline injection. The experimental conditions for this study are summarized in the following Table:
- Aniline Concentration: 0.1 M
- Running Solution: 0.5 M H2SO4
- CV Scan Rate: 100 mV/s
- CV Scan Potential Range: -0.2 to 0.8 V
Results